Ensure optimal throughput performance for cost-effective filtration
Most filter-intensive biotech processes like drug manufacturing require bioburden reduction from a broad range of fluid streams, buffers and wash liquids. Using the correct filtration system for reducing microbial contaminants in your bioproduction process is essential to drive efficiency and scalability to achieve drug critical quality.
Filtration costs can be well-managed by implementing robust highly efficient filters that reliably yield a high-quality filtrate. While both sterilizing-grade filters and dedicated bioburden control filters are effective, not all processes require a sterile filtrate. It is in these process steps that the higher flow rate delivered by bioburden control filters, due to a more open pore structure, can help to meet microbial safety objectives with greater efficiency.
Bioburden reduction filters can be used in preparative applications for:
- Growth media, buffers and reagents
- Removing post-cell harvest debris by depth filtration preceding the purification process
- Downstream intermediates and column guards
- Preparative filtration of chromatography and diafiltration buffers.
Consistent bioburden control in process streams can also help to protect and extend the service life of critical sterilizing-grade filters.
Scalable high-titer bioburden reduction
Our technologically innovative bioburden control filters reduce bacterial loads in process feeds to acceptably low levels for successful and cost-effective protection of processes from microbial and particulate contamination.
- Membranes are inert and have low protein and excipient binding properties suitable for all types of drug products.
- Available in a wide range of formats: cartridges for stainless steel installations and capsules for single-use systems.
- All process development-scale filter formats are designed to scale directly to larger cGMP filter configurations for fast and easy production scale-up.
A bioburden reduction step of <10 cfu /100 mL ahead of final sterile filtration is mandated by the European Medicines Agency. [1] It is our recommendation that qualitative and quantitative bioburden analysis be performed pre-sterile filtration.
Read more in our technical note: Should a pre-filtration bioburden be determined prior to sterile filtering a solution?
Supor EAV Bioburden Reduction Filters
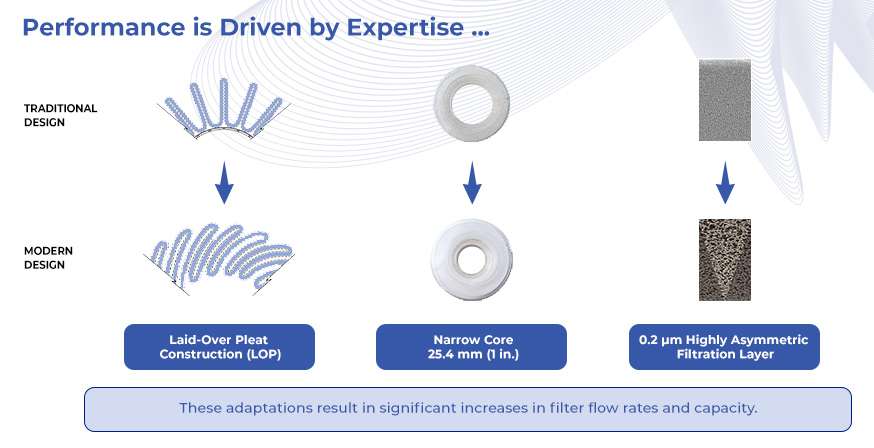
Supor® EAV filters have a 0.2 µm removal rating and feature a highly asymmetric, single-layer polyethersulfone (PES) membrane. These high-capacity filters ensure maximum flow and throughput performance:
- Smaller, more cost-effective filtration systems due to laid over, pleat membrane geometry, narrow core design and asymmetric membrane technology.
- Bacterial titer reduction in excess of 6 log for Brevundimonas diminuta guarantees excellent microbial filtration efficiency in processes with variable bioburden.
Depending on your validation needs, you can also choose to use a sterilizing-grade filter such as Supor EX ECV filters or Supor EKV filters for bioburden control.
Attribute guide:
| Family | Supor |
|---|---|
| Grade | EAV |
| Removal rating | 0.2 µm |
| Membrane material | PES |
Support, Drainage, Core Material |
Polypropylene |
| Gamma | Yes |
| Steam and autoclave | Yes |
| Compatibility | Good |
| Transmission (Preservatives/Proteins) | Excellent |
| Extractables | Good |
| Wettability after steam dry | No |
We understand you may be manufacturing several types of products which need different set-ups for each. This is why we’ve created a system that’s tailor made for different processes and drug entities. With our filtration solutions, you’ll be able to maximize your output, remove potential delays to your business, and ensure long-term efficiency.
Need help choosing the right filter?
Our Scientific and Laboratory Services (SLS) have been helping our customers optimize their processes for more than 50 years. Together with our wide range of filter membranes, we can find the right filtration solution for your fluid or help to optimize your sterile filtration process. Request a consultation with one of our sterile filtration specialists to discuss your process and free support to help you get it right.
Reference:
- EC, Eudralex Volume 4: EU Guidelines to Good Manufacturing Practice, Annex 1, Manufacture of Sterile Medicinal Products (Brussels, 2008).

Sterilizing-grade Liquid Filtration 0.2 µm Membranes