What happens to drinking water from its origin to the tap?
In most circumstances, well-controlled, hygienic water is delivered from water plants to cities. During transport, water is cold and flows continuously through large diameter pipes. However, this situation frequently changes at the point-of- entrance to buildings (1,2). Within buildings water can stagnate and its temperature increase. It passes through complex internal distribution systems consisting of narrow pipes with possibly corroded inner surfaces and dead ends. This environment can provide optimal conditions for the formation of biofilm from which bacteria and other microorganisms may be continuously released into the water (3-6).
DOWNLOAD IMAGE 1
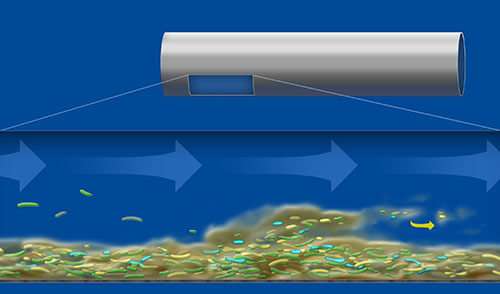
Image 1: Drinking water is derived from lakes, rivers or deep underground. It is purified in water plants and transported underground in large diameter pipes to cities and buildings, where it then often runs through small diameter pipes and may stagnate and warm up. These conditions are ideal for biofilm formation.
What is biofilm and how does it develop?
In water distribution systems biofilm can develop within a few days even if the water meets drinking water criteria (2). Biofilm can host bacteria, amoeba, algae and other microorganisms. Under low flow conditions, such as in dead legs, particularly thick biofilms can form. Under the force of waterflow biofilm can shear off and biofilm particles can colonize other parts of the water distribution system (3). External stress in the pipework, such as disinfection measures, can result in an increased expression of the biofilm phenotype cell which is responsible for the strong attachment of cells to a surface (5).
Image 2: Biofilm establishes in several phases over a few days. It contains microorganisms within its slimy matrix. With increasing thickness, biofilm particles containing large amounts of bacteria are released into the water stream.
Why does biofilm influence the water quality?
Biofilm protects the microorganisms within from chemical agents and thermal disinfection procedures (2,5). It is extremely difficult to completely eradicate the biofilm community once established. Irregular shedding from a biofilm can result in significant deviations of bacterial counts at sampling sites or points-of-use (POU) (2-4). Bacteria within biofilm communities have been shown to exhibit greater resistance against antimicrobial treatments than corresponding planktonic cells (3).
Image 3: When biofilm loaded with bacteria is released into the water stream, high microbial counts may be measured at the outlets. Annual testing provides only a snapshot of information, while regular testing is useful to monitor the bacterial risk of a pipe network.
Which micro- organisms can be found inside biofilms?
The majority of bacteria in a water pipework live within biofilm (about 95 %) and only about 5 % occur in
the water phase (7). Biofilms contain a large variety of waterborne microorganisms (91-93). These may include protozoa (e.g. Acanthamoeba), fungi (e.g. Aspergillus spp., Fusarium spp.), viruses and a number of human pathogenic bacteria (1,3,5,7-9). Among those bacterial species found in biofilm that are potentially harmful for immunocompromised people are Pseudomonas aeruginosa, non-tuberculous Mycobacteria, Stenotrophomonas maltophilia, Acinetobacter baumanii, Chrysobacterium spp., Sphingomonas spp., Aeromonas hydrophila, Simkania negevensis, Elizabethkingia meningoseptica and Klebsiella spp. (3,5,7,10-13). Legionella pneumophila is perhaps the best-known waterborne bacterium colonizing biofilm, and it can be found in both central storage areas (e.g. water tanks) as well as peripheral water outlets (2,3,5). Pseudomonas aeruginosa is known to be a major cause of severe infections (14-17), including pneumonia, sepsis, wound and skin infections.
Image 4: Biofilm in water networks may contain a large variety of microorganisms such as fungi (e.g. Aspergillus spp., left), rod-shaped bacteria (e.g. Legionella spp., middle) and protozoa (e.g. amoeba, right).
What are Viable But Non-Culturable cells?
The Viable But Non-Culturable (VBNC) cell fails to grow on routine bacteriological culture media, but is alive and capable of metabolic activity - indeed it can be “resuscitated” to a culturable state with renewed virulence (6, 18-21). This discovery has thrown the accuracy of quantifying culturing techniques into question. It is understood that a high proportion of biofilm dwelling cells live in the VBNC state and that the VBNC state can be induced by antibacterial material such as copper pipes (19), stress factors like starvation, exposure to temperatures outside the range of growth or exposure to chemical and thermal treatments (20-26). As water pathogens such as P. aeruginosa and L. pneumophila in their VBNC state are not detectable by standard culture methods, alternative diagnostic technologies such as Polymerase Chain Reaction (PCR), Fluorescence In Situ Hybridization (FISH) or determination of total cell number (TCN) are required in order to confirm their presence (6, 27). L. pneumophila, P. aeruginosa, Campylobacter jejuni and other waterborne bacteria have also been shown to be resuscitated to culturable cells under suitable stimuli (21, 28, 29).
Image 5: VBNC cells cannot be detected by classical culture methods.
What role do amoeba play in the biofilm community?
Amoeba are very important hosts for water bacteria. L. pneumophila, Mycobacteria spp. and other “amoeba resistant bacteria” can be carried by these protozoa (9, 30-34). Legionellae are taken up into amoeba without being digested and replicate there within vacuoles. When the Legionellae have reached a certain density, the vacuoles release them into the water system (31). Amoeba not only function as environmental reservoirs for Legionella, but have been also shown to be involved in selecting for, protecting and maintaining potentially pathogenic Legionella spp. in the environment (35, 36).
Image 6: Amoeba can incorporate Legionella which then proliferate inside vacuoles and are later released, either in the form of planktonic, free living bacteria, or packed within vacuoles.
Why is Pseudomonas aeruginosa of particular concern?
Image 7: Pseudomonas aeruginosa is an aerobic bacterial species and commonly found at the periphery of water systems such as taps, showers or sinks.
What are the possible infection pathways from water points-of-use to patients?
Inhalation and aspiration represent the established transmission pathways for Legionella spp. (49), whereas Pseudomonas spp. is reported to be mainly transmitted by contact and aspiration (17, 41). During daily routines, tap water is used for personal hygiene. For example, in the healthcare environment, due to the severity of their disease states, ICU patients often have multiple access devices such as catheters, drains and tracheal tubes. These portals represent potential entrance sites for bacteria from numerous sources. Droplets of contaminated tap water or contaminated hands of healthcare professionals can inadvertently come into contact with those entrance sites (50). Rogues et al. reported that 14 % of ICU healthcare professionals hands were Pseudomonas positive when washed with contaminated tap water and 12 % were positive when the last contact was with a Pseudomonas positive patient (41). Contaminated bottled water or contaminated water from drinking water dispensers has also been described as a source of Pseudomonas bacteria (51-53).
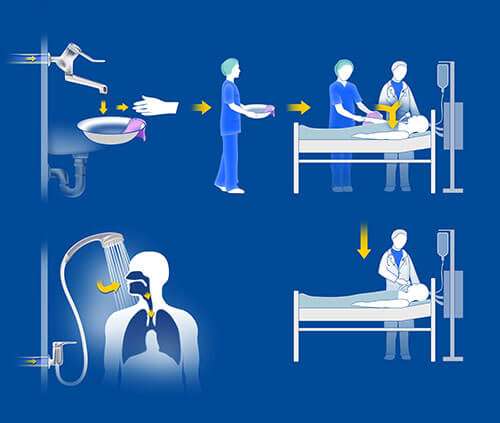
Image 8: Water for wound care, or the patient’s personal hygiene, may contain bacteria resulting in patient colonization and infection. A patient may not necessarily have to use a water outlet to become colonized; immobilized patients, e.g. within ICUs, can come in contact with contaminated water brought by health care workers to the patient’s bed.
Why is complete biofilm eradication by systemic treatments so difficult?
Water distribution systems in large buildings are frequently complex networks and can be up to 50 km in length. Dead ends, corroded pipes, low throughput, insuf cient temperature below 131 °F (55 °C) in the hot water pipes and above 68 °F (20 °C) in the cold pipes contribute to biofilm formation and impede eradication of biofilm (54). Heat & Flush procedures (e.g. 10-20 minutes of simultaneous flushing of all outlets with water heated to > 158 °F (> 70 °C) may have only short term effects (55). Legionella strains may even become heat resistant after thermal treatment over a long time (20). Moreover, the thermophilic Legionella community, including L. pneumophila, is able to grow in hot water system above 122 °F (50 °C) (56). Thermal procedures can result in warming up cold water (26, 57, 58), when both hot and cold water pipes are located in the same duct increasing the risk of biofilm in cold water. Chemical treatments are bactericidal to free floating bacteria but have limited effects on biofilm and may create hazardous byproducts during use (10, 55, 59, 60). Therefore, areas with vulnerable users may require additional protection (e.g. 0.2 μm point-of-use waterfiltration) to minimize risk of transmission of waterborne pathogens.
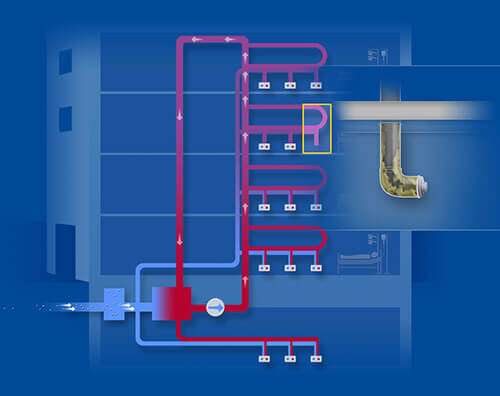
Image 9: Water systems in hospitals can contain corroded pipes and dead ends which cannot be reached by systemic disinfection. Bacteria can be released to recolonize the system after disinfection. Construction work may also cause bio lm release into the network.
Where are point-of-use (POU) water filters (tap filter, shower filter) typically used?
Point-of-use water filters can be used as an additional control measure in those areas where immunocompromised people may come into contact with water (61-85), in outbreak and critical contamination solutions as an aid in reducing patient exposure. They can be exibly installed at faucets (tap filters) or connected to shower hoses (shower filters). In medical facilities the most common areas for POU water filter installation are bone marrow transplant units, hematology/oncology units, ICUs, transplantation units, burn units, neonatology, endoscopic reprocessing, birthing pools, kitchen (for food preparation and drinking water provision), and geriatric departments. POU water filtration is also increasingly used in nursing homes or home care settings POU filters are quickly installed which makes them an effective and immediate management tool in acute water contamination situations.
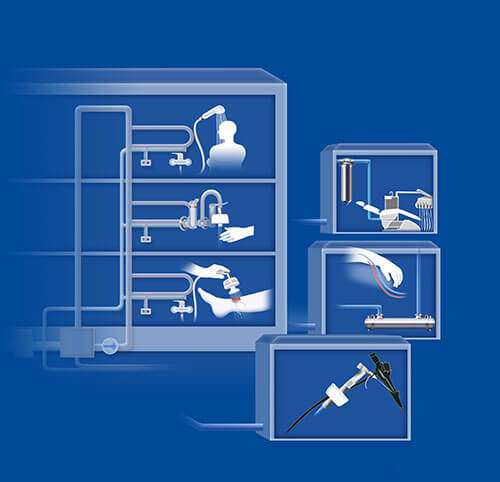
Image 10: Point-of-use water filters can be installed at taps, showers or in-line applications in various health care settings, such as hospitals, day surgery units, dental practices, dialysis units, or rehabilitation centers.
What are the user requirements for POU water filtration?
User facilities typically ask for POU filtration designed to deliver water quality in accordance with international standards for sterilizing grade filtration (complete retention of ≥107 CFU Brevundimonas diminuta/cm2 effective filtration area) (87-90). End point filters are mostly used in humid environments, and the risk of contamination of the filter housing exists through backsplash. In order to minimize this risk of retrograde contamination, Pall POU water filters contain anti-retrograde contamination technologies. Hygienic performance of these POU filters has been demonstrated through laboratory validation, multicentre field evaluations, and independent clinical studies demonstrating robust performance over the full product lifetime (61-85).
Image 11: F.e. the QPoint® Tap and Shower Assemblies consist of a permanent chrome docking station and a disposable, recyclable filter capsule for up to 62 days use. Advanced prefiltration technology improves ow rates throughout the filter capsule lifetime. An inner shield protects from direct back splash into the inner parts of the filter capsule.
Are there studies on POU water filtration?
Numerous reports have demonstrated the high efficiency of Pall’s POU water filters in the retention of microoganisms from water outlets in clinical locations and conditions (17, 28-49, 61-86).
References
- Cunliffe et al., “Water Safety in Buildings”, WHO Press, World Health Organization, Geneva/Switzerland, March, 2011
- Exner et al., “Prevention and control of health care-associated waterborne infections in health care facilities”, Am J Infect Control, 33:S26-S40, 2005
- Lindsay & von Holy, “Bacterial biofilm within the clinical setting: What healthcare professionals should know”, J Hosp Infect, 64:313-25, 2006
- Völker et al., “Modelling characteristics to predict Legionella contamination risk - Surveillance of drinking water plumbing systems and identification of risk areas”, Int J Hyg Env Health, 219:101-9, 2016
- Flemming & Wingender, “The biofilm matrix”, Nat Rev Microbiol, 8:623-33, 2010
- Inkinen et al., “Drinking water quality and formation of biofilms in an office building during its first year of operation, a full scale study”, Water Res, 49:83- 91, 2014
- Moritz et al., “Integration of Pseudomonas aeruginosa and Legionella pneumophila in drinking water biofilms grown on domestic plumbing materials”, Int J Hyg Env Health, 213:190-7, 2010
- Steinberg et al., “Adaption of Fusarium oxysporum and Fusarium dimerum to the specific aquatic environment provided by the water systems of hospitals”, Water Research 76:53-65, 2015
- Cateau et al., “Free-living amoebae. What part do they play in healthcare- associated infection?” J Hosp Inf, 87:131-40, 2014
- Falkinham et al., “Opportunistic premise plumbing pathogens: Increasingly important pathogens in drinking water”, Pathogens, 4(2):373-86, 2015
- Donati et al., “Prevalence of Simkania neqevensis in chlorinated water from spa swimming pools and domestic supplies”, J Appl Microbiol, 118(4):1016-82, 2015
- Narciso-da-Rocha et al., “Genotypic diversity and antibiotic resistance in Sphingomonadaceae isolated from hospital tap waters”, Sci Total Environ, 466:127-35, 2014
- Balm et al., “Bad design, bad practices, bad bugs: frustrations in controlling an outbreak of Elizabethkingia meningosephica in intensive care units”, J Hosp Infect, 85:135-40, 2013
- Anaissie et al., “The hospital water supply as a source of nosocomial infections: a plea for action”, Arch Intern Med, 162:1483-92, 2002
- Wunderink & Mendoza, “Epidemiology of Pseudomonas aeruginosa in the intensive care unit”, published in “Infectious diseases in critical care”, Springer Verlag, 218-25, 2007
- Trautmann et al., “Common RAPD pattern of Pseudomonas aeruginosa from patients and tap water in medical intensive care unit”, Int J Hyg Env Health, 209:325-31, 2006
- Loveday et al., “Association between healthcare water systems and Pseudomonas aeruginosa infections: a rapid systematic review”, J Hosp Infect, 86(1):7-15, 2014
- Oliver, “Recent findings on the viable but non-culturable state in pathogenic bacteria”, FEMS Microbiol Rev, 34:415-25, 2010
- Dwidjosiswojo et al., “Influence of copper ions on the viability and cytotoxicity of Pseudomonas aeruginosa under conditions relevant to drinking water environments”, Int J Hyg Env Health, 214(6):455-92, 2011
- Allegra et al., “Longitudinal evaluation of the efficacy of heat treatment procedures against Legionella spp. in hospital water systems by using a flow cytometric assay”, Appl Env Microbiol, 77:1268-75, 2011.
- Li et al., “The importance of the viable but non-culturable state in human bacterial pathogens”, Front Microbiol, 5:258, 2014
- Oliver, “The viable but nonculturable state in bacteria”, J Microbiol, 43:93-100, 2005
- Mustapha et al., “Monitoring of Legionella pneumophila viability after chlorine dioxide treatment using ow cytometry”, Res Microbiol, 166(3):215-9, 2015
- Epalle et al., “Viable not culturable forms of Legionella pneumophila generated after heat shock treatment are infectious for macrophage-like and alveolar epithelial cells after resuscitation on Acanthamoeba polyphaga”, Microbi Ecol, 69(1):215-24, 2015
- Baron et al., “Effect of monochloramine treatment on the microbial ecology of Legionella and associated bacterial populations in a hospital hot water system”, Syst Appl Microbiol, 38(3):198.205, 2015
- Mansi et al., “Legionella spp. survival after different disinfection procedures: Comparison between conventional culture, qPCR and EmA-qPCR”, Microchemical Journal, 112,65-9, 2014
- Flemming et al., “Microbial growth in drinking water distribution systems & tap water installations”, in van der Kooij D , van der Wielen P (eds) IWA Publishing, Chapter 8, 2013
- Pitkanen “Review of Campylobacter spp. in drinking and environmental waters”, J Microbiol Methods, 95:39-47, 2013
- Alleron et al., “VBNC Legionella pneumophila are suitable to produce virulence proteins”, Water Res, 47:6606-17, 2013
- Drancourt et al., “Interactions between Mycobacterium xenopi, amoeba and human cells”, J Hosp Infect, 65:138-42, 2007
- Pagnier et al., “Isolation of Vermamoeba vermiformis and associated bacteria in hospital water”, Microb Pathog, 80:14-20, 2015
- Zeybek & Binay, “Growth ability of gram negative bacteria in free-living amoeba”, Exp Parasitol, 145:121-6, 2014
- Cervero-Aragó et al., “Effect of common drinking water disinfectants , chlorine and heat, in free Legionella and Amoeba-associated Legionella”, PLOS one, 10(8):e0134726. Doi: 10.1371/journal.pone.0134726, 2015
- Drancourt, “Looking in amoeba as source of mycobacteria”, Microb Pathog, 77:119-24, 2014
- Lau & Ashbolt, “The role of biofilms and protozoa in Legionella pathogenesis: implications for drinking water”, J Appl Microbiol, 107(2):368-78, 2009
- Ashbolt, ”Environmental (Saprozoic) pathogens of engineered water systems: Understanding their ecology for risk assessment and management”, Pathogens, 4:390-405, 2015
- Vernier et al., “Risk factors for Pseudomonas aeruginosa acquisition in intensive care units: a prospective multicenter study”, J Hosp Inf, 88:103-8, 2014
- Cholley et al., “The role of water fittings in intensive care rooms as reservoirs for the colonization of patients with Pseudomonas aeruginosa”, Intensive Care Med, 34:1428-33, 2008
- Blanc et al., “Faucets as a reservoir of endemic Pseudomonas aeruginosa colonization/infections in intensive care units”, Intensive Care Med, 30:1964-8, 2004
- Vallés et al., “Patterns of colonization by Pseudomonas aeruginosa in intubated patients: a 3-year prospective study of 1,607 isolates using pulsed-field gel electrophoresis with implications for prevention of ventilator-associated pneumonia”, Intensive Care Med, 30:1768-75, 2004
- Rogues et al., “Contribution of tap water to patient colonisation with Pseudomonas aeruginosa in a medical intensive care unit”, J Hosp Infect, 67:72-8, 2007
- Falkinham et al., “Epidemiology and ecology of opportunistic premise plumbing pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa”, Environ Health Perspect, 123(8):749-58, 2015
- Walker et al., “Investigation of healthcare acquired infections associated with Pseudomonas aeruginosa biofilms in taps in neonatal units in Northern Irland”, J Hosp Infect, 86(1):16-23, 2014
- Walker & Moore, “Pseudomonas aeruginosa in hospital water systems: biofilms, guidelines prachalities”, J Hosp Inf, 89(4):324-27, 2015
- Kumar et al., ”Incidence rate of multidrug-resistant organisms in a tertiary care hospital, North Delhi”, Apollo Medicine, 12(4):248-256,2015
- Vaz-Moreera et al., “Diversity and antibiotic resistance in Pseudomonas spp. from drinking water”, Sci Total Environ, 426:366-74, 2012
- Suman et al., “Pseudomonas aeruginosa biofilms in hospital water systems and the effect of sub-inhibitory concentrations of chlorine”, J Hosp Infect, 70(2): 199-201, 2008
- Simon et al., “Anforderungen an die Hygiene bei der medizinischen Versorgung von Patienten mit Cystischer Fibrose (Mukoviszidose) ”, Empfehlungen auf Anregung der Kommission für Krankenhaushygiene und Infektionsprävention beim Robert Koch-Institut, mhp Verlag, 2012
- Hines et al., “Assessment of relative potential for Legionella species or surrogates enhances exposure from common water sources”, Water Res, 56:203-13, 2014
- Tajeddin et al., “The role of the intensive care unit environment and health care workers in the transmission of bacteria associated with hospital acquired infections”, J Infect Public Health, pii: S1876-0341(15)00107-0. doi: 10.1016/ j.jiph.2015.05.010, 2015
- Eckmanns et al., “An outbreak of hospital-acquired Pseudomonas aeruginosa infections caused by contaminated bottled water in intensive care units”, Clin Microbiol Infect, 14:454-8, 2008
- Wong et al., “Spread of Pseudomonas fluorescens due to contaminated drinking water in a bone marrow transplant unit”, J Clin Microbiol, 49:2093-6, 2011
- Costra et al., “Nosocomial outbreak of Pseudomonas aeruginosa associated with a drinking water fountain”, J Hosp Infect, 91:271-4, 2015
- Bédard et al., “Temperature diagnostic to identify high risk areas and optimize Legionella pneumophila surveillance in hot water distribution systems”, Water Res, 71:244-56, 2015
- Blanc et al., “Water disinfection with ozone, copper and silver ions, and temperature increase to control Legionella: seven years of experience in a university teaching hospital”, J Hosp Infect 60:69-72, 2005
- Lesnik et al., “Legionella species diversity and dynamics from surface reservoir to tap water: from cold adaptation to thermophily”, The ISME Journal, doi:10.1038/ ismej.2015.199
- Arvand et al., “Contamination of the cold water distributor system of health care facilities by Legionella pneumophila: Do we know the true dimension?”, Euro Surveill, 16(6):pii:19844, 2011
- “Legionella and the prevention of Legionellosis”, WHO Press, World Health Organization, Geneva/Switzerland, Editors: J. Bartram, Y. Chartier, JV Lee, K. Pond & S. Surman-Lee, 2007
- Eckmanns et al., “Prevention of nosocomial Legionnaire’s disease”, Dtsch Ärztebl, 103:1294-300, 2006
- Lin et al., “Controlling Legionella in hospital drinking water: An evidence-based review of disinfection methods”, Infect Control Hosp Epidemiol, 32:166-73, 2011
- Vianelli et al., “Resolution of a Pseudomonas aeruginosa outbreak in a haematology unit with the use of disposable sterile water filters”, Haematologica, 91:983-5, 2006
- Van der Mee-Marquet et al., “Water Microfiltration: A procedure to prevent Pseudomonas aeruginosa infection”, XVIe Congrès National de la Société Francaise d’Hygiène Hospitalière, Reims, Livre des Résumés, S137, June 4th 2005
- Hall et al., “Provision of safe potable water for immunocompromised patients in hospital”, J Hosp Infect, 58:155-8, 2004units. Department of Health Gateway Ref 17334, 2012
- Trautmann et al., “Point-of-use water filtration reduces endemic Pseudomonas aeruginosa infections on a surgical intensive care unit”, Am J Infect Control, 36:421-9, 2008
- Legrand et al., “Impact of terminal water microfiltration on the reduction of nosocomial infections due to Pseudomonas aeruginosa in burnt patients”, presented at 11ème Journées Internationales de la Qualité Hospitalière et en Santé (JIQHS), Paris, poster n°204, 2009
- Wright et al., “A two year double cross-over study investigating point-of-use filters for reducing Gram-negative nosocomial pathogens from hospital water”, J Hosp Infect 76(Suppl. 1):S38, 2010
- Hell et al., “Water micro ltration at the point of use – a procedure to prevent infection/colonization with water borne pathogens in ICU patients?”, J Hosp Infect, 76(Suppl. 1):S38, 2010
- Rolling, “Handling of antimicrobial water lter and disposable shower rose used for wound cleansing”, 19th Conf European Wound Management Assoc, Helsinki, Finland, (P27), May 2009
- Barna et al., “Infection control by point-of-use water ltration in an intensive care unit – a Hungarian case study”, J Water Health, 12(4):858-67, 2014
- Warris et al., “Point-of-use ltration method for the prevention of fungal contamination of hospital water”, J Hosp Infect, 76:56-9, 2010.
- Junker et al., “Nosocomial infections with Legionella spp. and other water borne bacteria can be reduced by control of shower water”, Int J Infect Contr, 5:S60 (P17), 2009
- Williams et al., “Point-of-use membrane ltration and hyperchlorination to prevent patient exposure to rapidly growing mycobacteria in the potable water supply of a skilled nursing facility”, Infect Contr Hosp Epidemiol, 32:837-44, 2011
- Wang et al., “Pseudo-outbreak of Mycobacterium paraf nicum infection and/ or colonization in a tertiary care medical center”, Infect Contr Hosp Epidemiol, 30:848-53, 2009
- Harpel et al., “Performance of a new 14 day water lter during daily use in clinical routine at two university medical centers”, J Hosp Infect, 64 (Suppl.1):S47, 2006
- Sheffer et al., “Ef cacy of new point-of-use water lter for preventing exposure to Legionella and waterborne bacteria”, Am J Infect Control, 33:S20-S25, 2005
- Warris et al., “Point-of-use ltration method for the prevention of fungal contamination of hospital water”, J Hosp Infect, 76:56-9, 2010.
- Cervia et al., “Point-of-use water ltration reduces healthcare-associated infections in bone marrow transplant recipients”, Transpl Infect Dis, 12:238-41, 2010
- Holmes et al., “Preventive ef cacy and cost-effectiveness of point-of-use water ltration in a subacute care unit”, Am J Infect Control, 38:69-71, 2010
- Williams et al., “Point-of-use membrane ltration and hyperchlorination to prevent patient exposure to rapidly growing mycobacteria in the potable water supply of a skilled nursing facility”, Infect Contr Hosp Epidemiol, 32:837-44, 2011
- Hankwitz et al., “Non-tuberculous mycobacterial hypersensitivity pneumonitis related to a home shower: treatment and secondary prevention”, BMJ Case Reports, doi: 10.1136/bcr.06.2011.4360, 2011
- Zhou et al., “Removal of waterborne pathogens from liver transplant unit water taps in prevention of healthcare-associated infections: a proposal for a cost- effective, proactive infection control strategy”, Clin Microbiol Infect, 20: 310–4, 2014
- Teare & Millership, “Legionella pneumophila serogroup 1 in a birthing pool”, J Hosp Infect, 82:58-60, 2012
- Aumeran et al., “Pseudomonas aeruginosa and Pseudomonas putida outbreak associated with contaminated water outlets in an oncohaematology paediatric unit”, J Hosp Infect, 65(1):47-53, 2007
- Litvinov et al., “An outbreak of invasive fusariosis in a children’s cancer hospital”, Clin Microbiol Infect, 21(3):268.e1-7, 2015
- Wei et al., “Nosocomial neonatal legionellosis associated with water in infant formula, Taiwan”, Emerg Infect Dis, 20(11):1921-4, 2014
- Demirjian et al., “The importance of clinical surveillance in detecting Legionnaires’ disease outbreaks: a large outbreak in a hospital with a Legionella disinfection system-Pennsylvania, 2011-2012”, Clin Infect Dis, 60(11):1596-1602, 2015
- American Standard Testing Method (ASTM) F838-15a “Determining Bacterial Retention of Membrane Filters Utilized for Liquid Filtration”, 2015
- FDA, “Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing – Current Good Manufacturing Practice”, September 2004, http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070342.pdf
- HIMA Document No.3, Vol 4 “Microbiological evaluation of lters for sterilising liquids”
- European Pharmacopoeia, 8th Edition, January 2014, https://www.edqm.eu/ en/european-pharmacopoeia-8th-edition-1563.html
- Falkinham, JO “Nontuberculous mycobacteria: Community and nosocomial waterborne opportunistic pathogens”, Clinical Microbiology Newsletter, 38(1):1- 7, 2016
- Bautista-de los Santos, QM, et al., “Emerging investigators series: Microbial communities in full-scale drinking water distribution systems - a meta-analysis”, Environmental Science: Water Reesearch & Technology, 2(4):631-644, 2016
- Bautista-de los Santos, QM, et al., “The impact of sampling, PCR, and sequencing replication on discerning changes in drinking water bacterial community over diurnal time-scales”, Water Research, 90:216-24, 2016