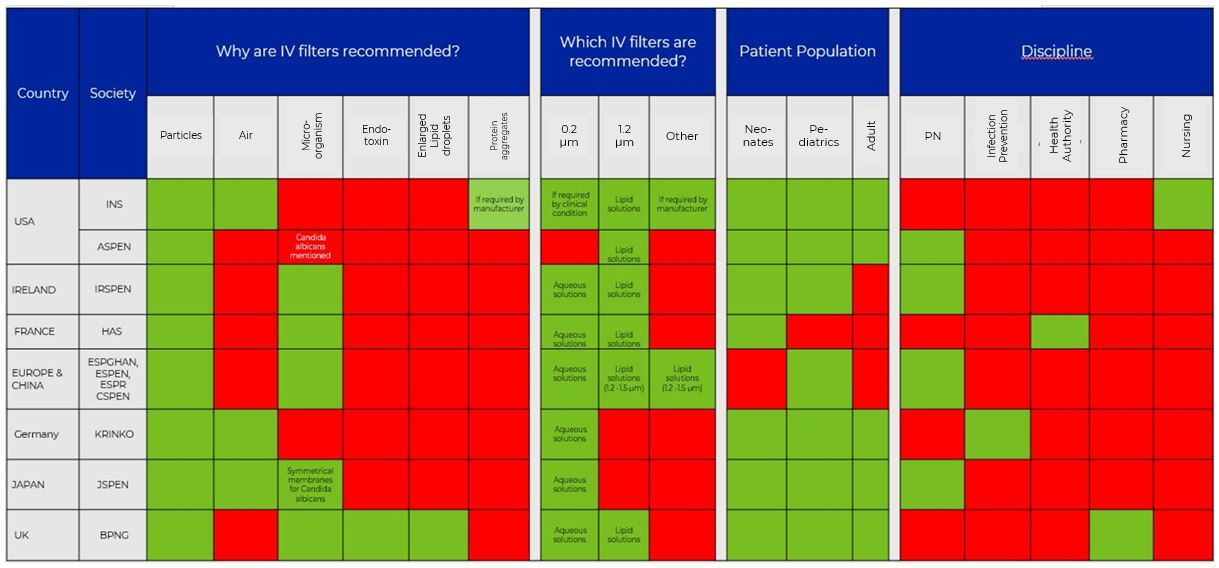
Learn how IV filter recommendations differ between clinical societies.
"Clinical guidelines are meant to help ensure that patients receive appropriate treatment and care. Guidelines summarize the current medical knowledge, weigh the benefits and harms of diagnostic procedures and treatments, and give specific recommendations based on this information. They should also provide information about the scientific evidence supporting those recommendations (1)."
Table 1. Guidelines recommending IV in-line filters
| Country | Society | Year | |
|---|---|---|---|
| USA | INS | Infusion Nursing Society | 2021 |
| ASPEN | American Society of Parenteral and Enteral Nutrition | 2020 |
|
| Ireland | IRSPEN | Irish Society for Clinical Nutrition & Metabolism | 2020 |
| France | HAS |
Haute Autorité De Santé |
2018 |
| Europe and China | ESPGHAN |
European Society for Paediatric Gastroenterology Hepatology Nutrition |
2018 |
| ESPEN |
European Society for Parenteral and Enteral Nutrition |
||
| ESPR |
European Society for Paediatric Research |
||
| CSPEN |
Chinese Society for Parenteral and Enteral Nutrition |
||
| Germany | KRINKO |
Commission for Hospital Hygiene and Infection Prevention |
2017 |
| Japan | JSPEN | Japanese Society for Clinical Nutrition and Metabolism | 2013 |
| UK | BPNG | British Pharmaceutical Nutrition Group | 2001 |
After the occurrence of two deaths and at least two cases of respiratory distress related to calcium phosphate precipitation, the Food and Drug Administration (FDA) suggested in 1994, the use of a 1.2 micron air-eliminating filter for lipid-containing products and a 0.22 micron air-eliminating filter for lipid-free products (2). In the following 30 years, several national and international organizations developed their own recommendations by reviewing the increasing scientific evidence regarding the potential benefits of IV filters.
This blog summarizes the current situation around the globe by comparing eight guidelines and their recommendations regarding the usage of IV in-line filters.
What is the situation in the US regarding the recommendations of IV filters? Keep it simple
In the US the Infusion Nursing Society (INS) and the American Society for Parenteral and Enteral Nutrition (ASPEN) are advocating the use of IV in-line filters.
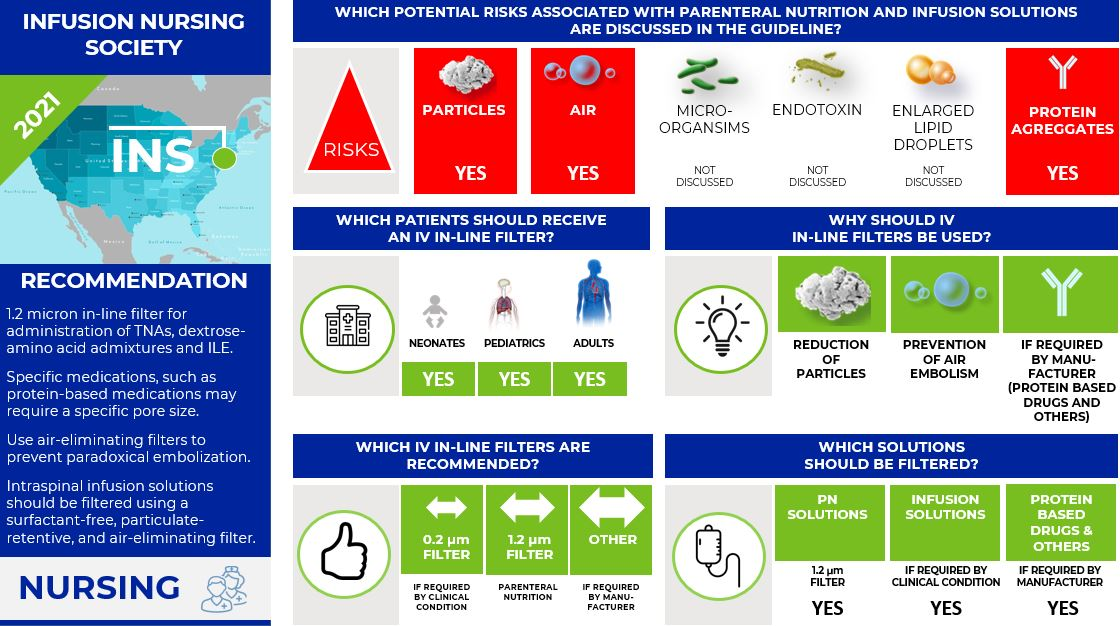
Infusion Nurses Society (INS) (3,4)
To align with ASPEN, the INS published in 2021 the "Infusion Therapy Standards of Practice Updates". This new recommendation supersedes the "8th Edition of the Infusion Therapy Standards of Practice" for the use of 0.22-micron filtration for non-lipid solutions in parenteral nutrition published in the same year. For the same reason as ASPEN (reducing the risk of errors associated with using two different types of filters), INS is now recommending using only a 1.2 micron in-line filter for the administration of TNAs, dextrose-amino acid admixtures, and intravenous lipid emulsions (ILEs).
Beside this new recommendation for parenteral nutrition, INS states in the "8th Edition of the Infusion Therapy Standards of Practice":
- Use the appropriate pore size in-line filter as required by the specific solution or medication to be infused. Consult with pharmacy for specific medication information.
- Some medications may require a specific pore size due to the molecular size of the medication (e.g., amphotericin B) and/or the concentration for infusion (e.g., mannitol).
- Recommendations for filtration of protein-based medications (e.g., immunoglobulin, monoclonal antibodies, enzymes) vary greatly, including many drugs with no filtration instructions and many variations in filter pore size recommended. Many protein-based medications indicate the need for "low protein binding filters," which include filters made of polyethersulfone, polyvinylidene fluoride, and cellulose acetate.
- Use air-eliminating filters for infusion in all patients with a medical diagnosis involving right-to-left cardiac or pulmonary shunting to prevent air and particulate matter from reaching the arterial circulation, also known as paradoxical embolization.
- Intraspinal infusion solutions should be filtered using a surfactant-free, particulate-retentive, and air-eliminating filter.
Infographic. INS IV filter recommendations.
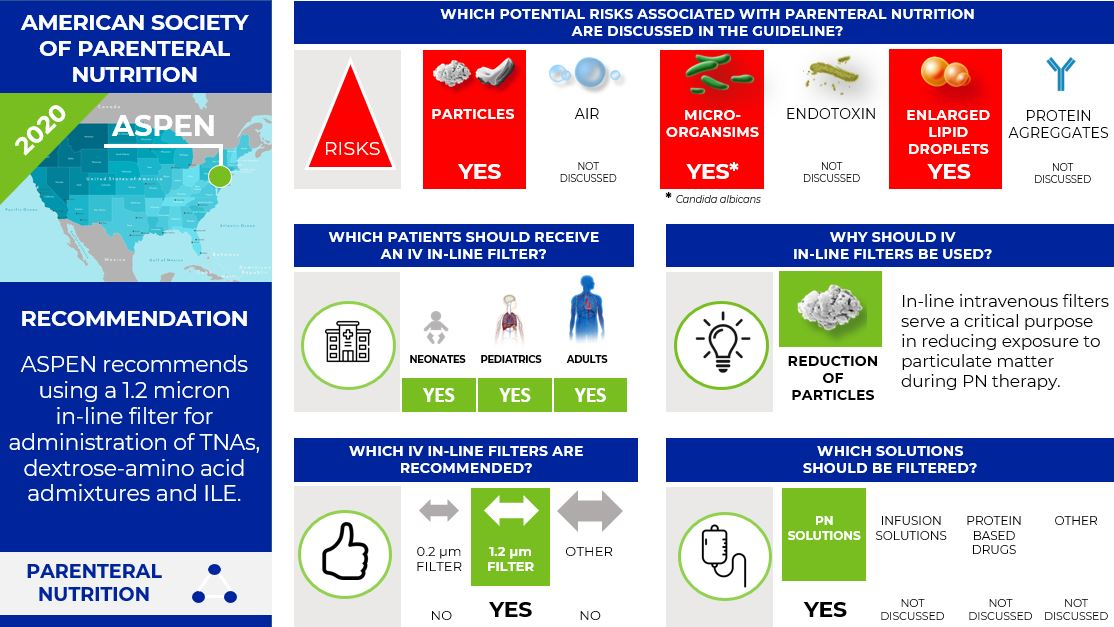
The American Society for Parenteral and Enteral Nutrition (ASPEN) (5)
The 2014 ASPEN PN Safety Consensus Recommendations stated to use a 0.22-micron filter for dextrose-amino acid admixtures and a 1.2-micron filter for TNAs. To increase compliance with recommendations for filter use with PN administration the 2020 ASPEN Position Paper simplifies this recommendation and recommends now using only a 1.2 micron in-line filter for administration of total nutrient admixtures (TNAs), dextrose-amino acid admixtures, and intravenous lipid emulsions (ILEs) instead of using two filters with different pore sizes. The goal is to alleviate the potential confusion and errors associated with using two IV in-line filters.
ASPEN recommends that healthcare organizations that do not filter PN admixtures or ILE reevaluate these decisions and consider the small price of filters in comparison to increased morbidity and mortality that may result from not filtering ILE or PN.
Further, ASPEN states:
- "In-line intravenous filters serve a critical purpose in reducing exposure to particulate matter during PN therapy. Particles greater than 2 microns, which are retained by 1.2 micron filters, appear to pose the most serious risk for adverse consequences."
- "Although 1.2 micron filters are not recommended for use as a routine infection control measure, ASPEN states that these devices are effective in preventing Candida albicans, a pathogen frequently associated with PN administration, from reaching the patient."
- "Numerous factors such as pH, temperature, and the concentration of macronutrients, divalent cations, trivalent cations, and free fatty acids can cause ILE droplets to enlarge in TNAs, leading to coalescence of the droplets and, eventually, the release of free oil into the admixture."
Infographic. ASPEN IV filter recommendations.
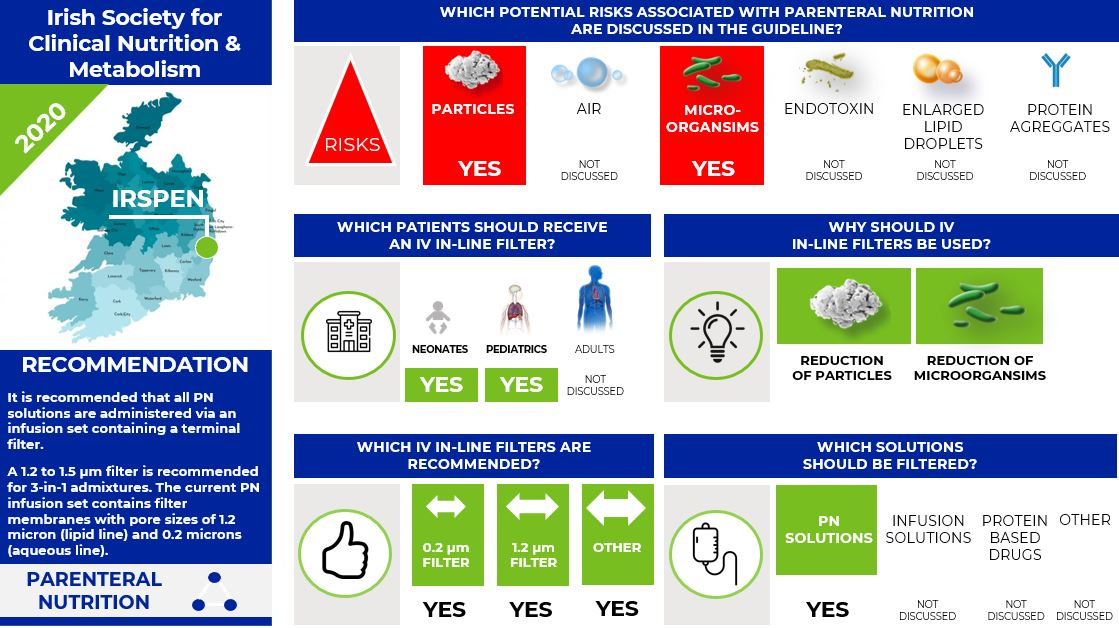
What is the situation in Ireland? Focus on neonates and pediatrics (6)
In 2020, the Irish Society for Clinical Nutrition & Metabolism (IRSPEN) published the Guideline on the use of Parenteral Nutrition in Neonatal and Pediatric Units. The IRSPEN states that PN solutions may contain particulate matter and biochemical interactions can result in chemical precipitations in addition to the risk of bacterial contamination.
The IRSPEN states:
- It is recommended that all PN solutions are administered via an infusion set containing a terminal filter.
- A 1.2 to 1.5 micron filter is recommended for 3-in-1 admixtures.
The current PN infusion set contain filter membranes with pore sizes of 1.2 micron (lipid line) and 0.2 microns (aqueous line).
Infographic. IRSPEN IV filter recommendations.
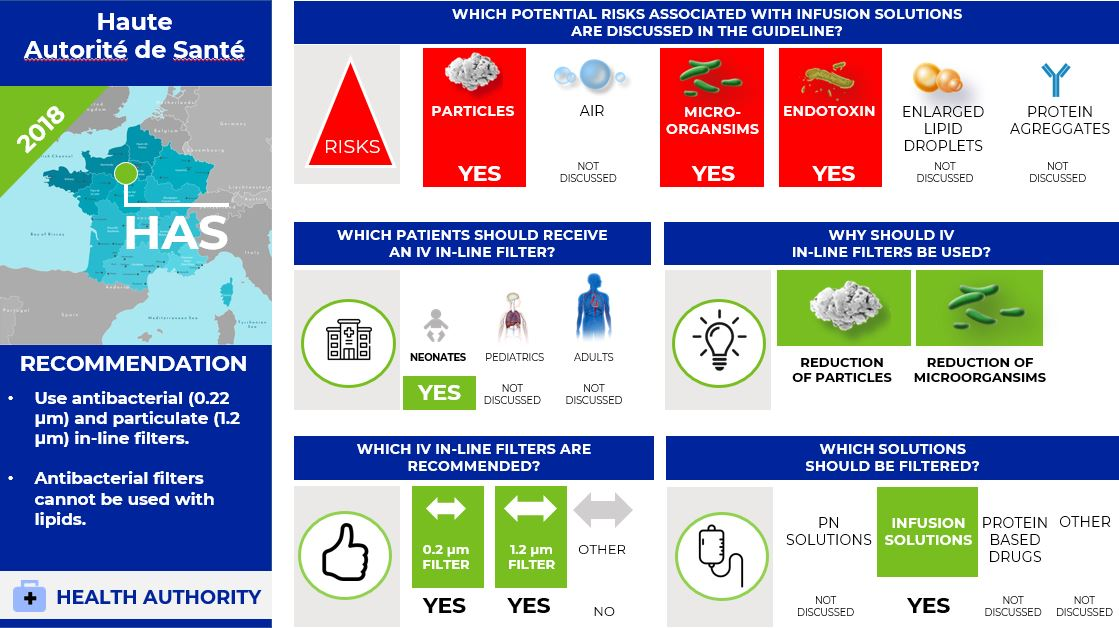
What is the situation in France? Focus on neonates (7)
In 2018, the French Health Authority "Haute Autorité de Santé" (HAS) published guidelines on the use of PN in neonates.
The HAS recommends using antibacterial (0.22 µm) and particulate (1.2 µm) in-line filters. Further, the HAS states that antibacterial filters cannot be used with lipids.
Infographic. HAS IV filter recommendations.
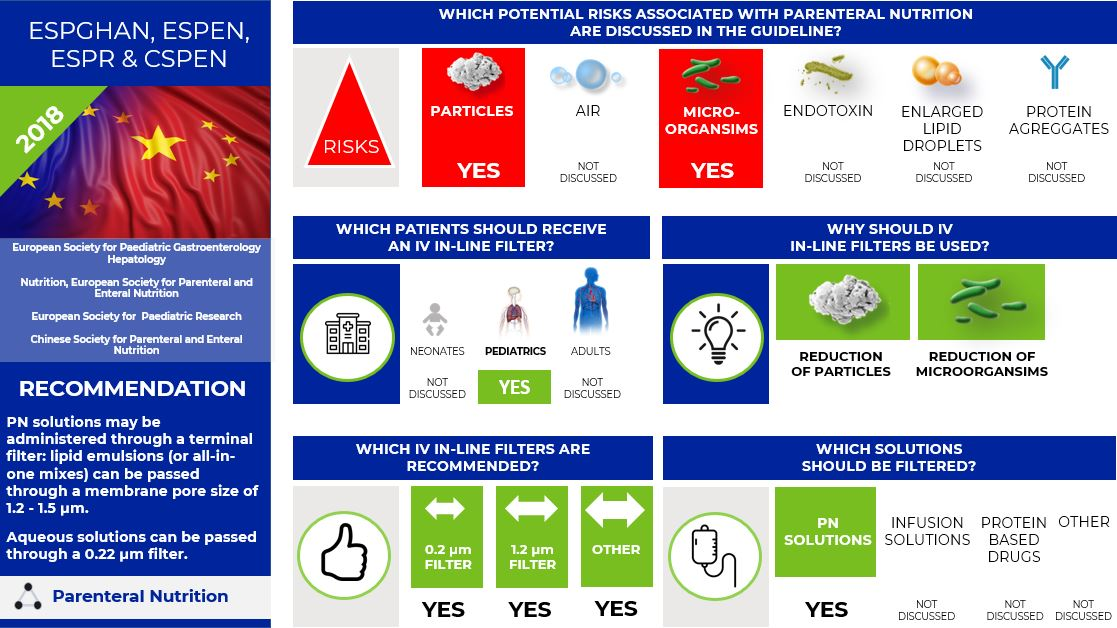
What is the situation in Europe and China? Focus on pediatric parenteral nutrition (8)
In 2018, the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN), European Society for Parenteral and Enteral Nutrition (ESPEN), European Society for Paediatric Research (ESPR), and Chinese Society for Parenteral and Enteral Nutrition (CSPEN) working group published new guidelines on pediatric parenteral nutrition including recommendation on the IV in-line filters for PN.
The guideline states that PN solutions contain particulate matter and biochemical interactions can lead to chemical precipitates and emulsion instability; they also act as a media for microbiologic growth should contamination occur.
The guideline recommends:
- PN solutions may be administered through a terminal filter: lipid emulsions (or all-in-one mixes) can be passed through a membrane pore size of 1.2 - 1.5 µm.
- Aqueous solutions can be passed through a 0.22 µm filter.
Infographic. ESPGHAN, ESPEN, ESPR, and CSPEN IV filter recommendations.
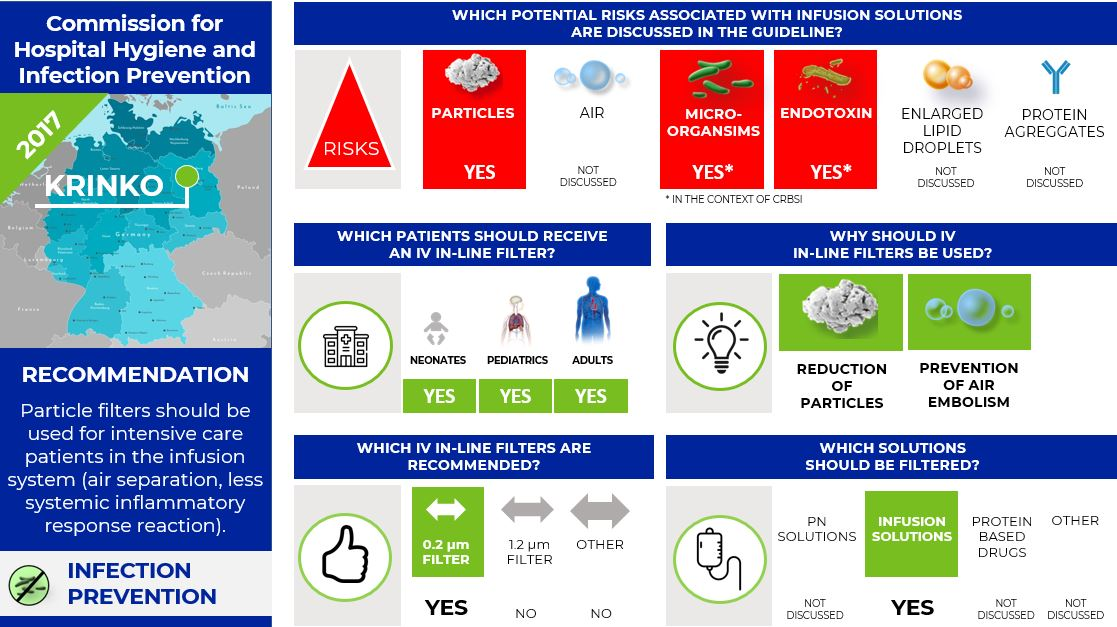
What is the situation in Germany? The infection preventionist guideline (9)
The Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch-Institute in Germany published the guidelines "Prevention of infections from vascular catheters" in 2017. In the chapter "Bacteria- and Endotoxin filter" the commission recommends using IV in-line filter for the treatment of intensive care unit patients for the prevention of particles and air.
- Particle filters should be used for intensive care patients in the infusion system (air separation, less systemic inflammatory response reaction).
- 0.2 µm filters (bacterial filters) for the prevention of CRBSI are not recommended.
Infographic. KRINKO IV filter recommendations.
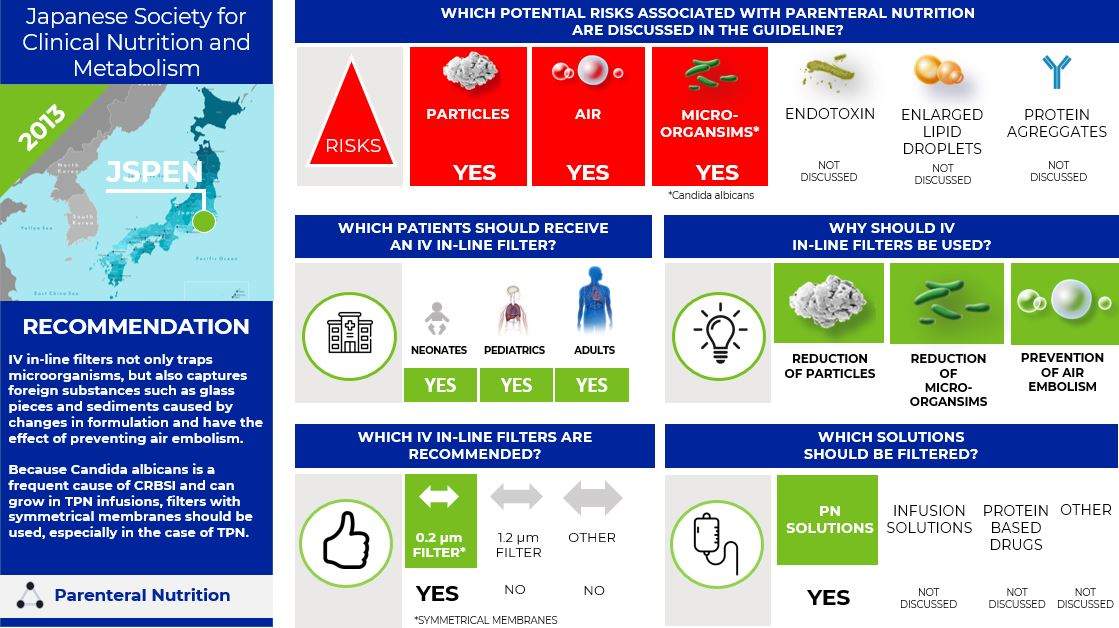
What is the situation in Japan? Asymmetrical vs. symmetrical membranes (10)
In 2013 the Japanese Society for Clinical Nutrition and Metabolism (JSPEN) published the guideline "Parenteral and Enteral Nutrition Guidelines Third Edition".
The JSPEN states that there is no evidence that IV filters reduce CRBSI, but they could remove fungi and bacteria. Currently, the 0.22 μm in-line filters used in Japan are divided into symmetrical membranes, in which the inflow and outflow are equally structured, and asymmetric membranes, in which the pore size of the inflow is larger. Both are standard for 0.22 μm filters, but it was shown that Candida albicans could penetrate 0.22 μm filters of asymmetric membranes (11). It has also been experimentally demonstrated that a 0.22 μm filter consisting of a symmetrical membrane can block Candida albicans penetration for 168 hours (12).
The JSPEN states that
- IV in-line filters not only trap microorganisms, but also capture foreign substances such as glass pieces and sediments caused by changes in formulation and have the effect of preventing air embolism.
Because Candida albicans is a frequent cause of CRBSI and can grow in TPN infusions, filters with symmetrical membranes should be used, especially in the case of TPN.
Infographic. JSPEN IV filter recommendations.
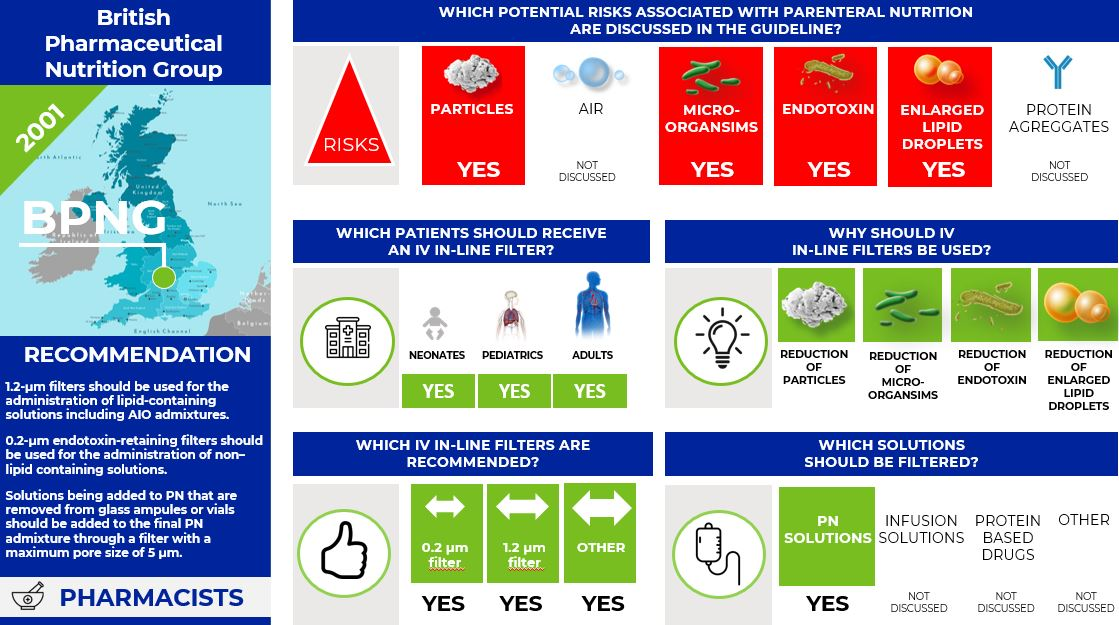
What is the situation in the United Kingdom? The pharmacist guideline (13)
In 2001 the British Pharmaceutical Nutrition Group (BPNG) commissioned a panel of experts on the stability and filtration of PN to evaluate the current evidence about filtration and develop recommendations for filter usage with PN.
The BPNG states that
- appropriate filters should be used during the administration of PN to patients who require intensive or prolonged parenteral therapy, the immunocompromised, neonates and children, and patients receiving home PN because of the large volume of potentially particulate-contaminated fluid administered and their increased susceptibility to the detrimental effects of particulate contamination.
- 1.2-µm filters should be used for the administration of lipid-containing solutions including AIO admixtures and changed every 24 h.
- 0.2-µm endotoxin-retaining filters should be used for the administration of non–lipid containing solutions and can be changed every 96 h.
- solutions being added to PN that are removed from glass ampules or vials should be added to the final PN admixture through a filter with a maximum pore size of 5 µm, to reduce the particle load from extrinsic and intrinsic contamination introduced from raw materials during PN production.
Infographic. BPNG IV filter recommendations.
Summary
Over the last years, several societies, mainly parenteral nutrition societies, made recommendations regarding the use of IV in-line filters. Interestingly, the situation is not as clear or uniform as expected, and the recommendations vary widely from society to society.
In a nutshell:
- Overall, particulate matter (followed by air and microorganisms) seem to drive IV in-line filter recommendations.
- 5 in 8 guidelines recommend using 0.2 µm and 1.2 µm IV in-line filters.
- 4 in 8 guidelines are written by Parenteral Nutrition Societies.
- Only 1 in 8 guidelines evaluates the risk of protein aggregates and refers to the manufacturer’s instructions for use regarding correct IV in-line filter selection.
- Only 1 in 8 guidelines evaluates the risk of endotoxin and enlarged lipid droplets as a reason for using an IV in-line filter.
- 0 in 8 guidelines consider all potential risks of infusion management, such as particulate matter, air, microorganisms, endotoxin, enlarged lipid droplets, and protein aggregates.
The global situation seems to be confusing, and one can only hope that guidelines become uniform around the globe in the future. Of course, barriers to physician and nurse’s adherence to practice guidelines may also exist for the usage of IV in-line filters. These barriers may include lack of awareness, lack of familiarity, lack of agreement, lack of self-efficacy, and lack of outcome expectancy (14).
But this is another story.
References
- InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficient in Health Care (IQWiG). What are clinical practice guidelines? 2016 Jun 15 [Updated 2016 Sep 8]. https://www.ncbi.nlm.nih.gov/books/NBK390308/
- Drug Information Group, College of Pharmacy, University of Illinois Chicago [Internet]. What are current recommendations for filtering parenteral nutrition (PN) admixtures? 2021. https://dig.pharmacy.uic.edu/faqs/2021-2/august-2021-faqs/what-are-current-recommendations-for-filtering-parenteral-nutrition-pn-admixtures
- Gorski LA, Hadaway L, Hagle ME, et al. (2021). Infusion therapy standards of practice. J Infus Nurs. 2021;44(suppl 1): S1-S224. doi:10.1097/NAN.0000000000000396
- 2021 Infusion Therapy Standards of Practice Updates. J Infus Nurs. 2021 Jul-Aug;44(4): 189-190. doi: 10.1097/NAN.0000000000000436.
- Worthington P, Gura KM, Kraft MD, Nishikawa R, Guenter P, Sacks GS. et al. Update on the Use of Filters for Parenteral Nutrition: An ASPEN Position Paper. Nutr Clin Pract. 2021; 36(1):29-39. doi.10.1002/ncp.10587.
- Irish Society for Clinical Nutrition & Metabolism. 2020. Guideline on the Use of Parenteral Nutrition in Neonatal and Paediatric Units. Revision date: 2023 Jul. Version 2.0. Guideline no. CSPD001/2017. https://www.hse.ie/eng/about/who/cspd/ncps/paediatrics-neonatology/resources/guideline-on-the-use-of-parenteral-nutrition-in-neonatal-and-paediatric-units.pdf
- Haute Autorité de Santé. Recommandation de bonne pratique. Nutrition parentérale en néonatologie. French. 2018 Apr 4. https://www.has-sante.fr/jcms/c_2859140/en/nutrition-parenterale-en-neonatologie-recommandation-de-bonne-pratique
- Puntis JWL, Hojsak I, Kziazyk J, ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Organisational aspects. Clin Nutr. 2018;37(6 Pt B):2392-2400. doi:10.1016/j.clnu.2018.06.953.
- Prävention von Infektionen, die von Gefäßkathetern ausgehen: Teil 1 – Nichtgetunnelte zentralvenöse Katheter Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2017 Feb;60(2):171-206. German.
- Japanese Society for Clinical Nutrition and Metabolism. 2013. Japanese.
- Inoue Y. The Japanese Journal of Surgical Metabolism and Nutrition. 2008;42:11-18.
- Inoue Y. et al. The Japanese Journal of Surgical Metabolism and Nutrition. 2006;40:229-237.
- Bethune K, Allwood M, Grainger C, Wormleighton C, British Pharmaceutical Nutrition Group Working Party. Use of filters during the preparation and administration of parenteral nutrition: position paper and guidelines prepared by a British pharmaceutical nutrition group working party. Nutrition. 2001;17(5):403-408. doi:10.1016/s0899-9007(01)00536-6.
- Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465. doi:10/1001/jama.282.15.1458.
Author bio
Dr. Volker Luibl, MBA

Dr. Luibl is Demand Generation Marketing Manager in medical device and clinical science.