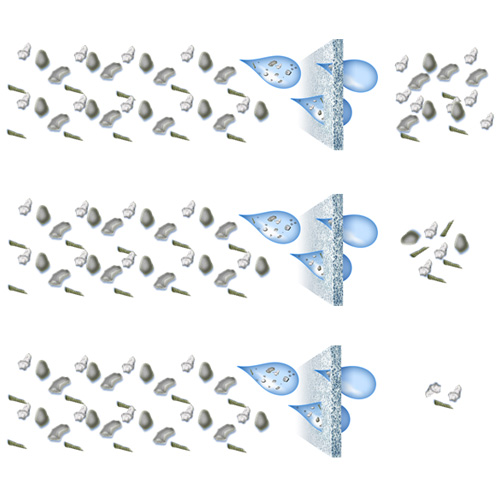
The world of filtration is more complicated than it seems. It can appear likely that at 1 micron, a filter, A, has the same performance as another filter, B. It is often assumed that both filters trap all particles larger than 1 micron, but this is not necessarily the case. The micron size of a filter only tells part of the story about the filter’s performance.
In potable water filtration, there are categories that help describe a filter’s performance. For example, a crude filter (or strainer) can be considered to have the ability to catch some small number of particles at a given micron size. A nominal filter typically can retain 50%–90% of particles at a given size (1). An absolute filter should retain 99.9% of particles at a defined size, and this can be confirmed using a glass bead challenge or other test method. Filter efficiency is sometimes expressed as a beta ratio, β, where the particles upstream of a filter are divided by the particles downstream of a filter. The higher the number, the more efficient the filter (2). For waterborne organisms, a microbial filter should provide a log reduction in bacteria according to ASTM F838, and a sterilizing-grade filter should show no bacterial recovery when tested to ASTM F838 (3,4).
As an example, let us assume we have a crude, a nominal, an absolute, a microbial, and a sterilizing grade filter all rated at 0.2 microns. For a crude, nominal, and absolute filter, let us also assume that we have 100 particles in a water sample. After the water passes through the filter, we might see as many as the following particles pass through:
- Crude: 99 particles
- Nominal: 50 particles
- Absolute: < 0.1 particles
Fig 1. From top to bottom, examples of crude, nominal, and absolute filtration.
However, waterborne organisms are living things with differing shapes and sizes. Therefore, an absolute filter may not be sufficient to retain organisms even at 0.2 microns. As mentioned above, absolute filters are tested with spherical, uniform glass beads and microbial/sterilizing-grade filters are tested with bacteria so the results cannot be compared.
In the blog post on ASTM F838 microbial and sterilizing-grade filters were compared. The following example was shared to illustrate that a microbial filter’s performance can be much different than sterilizing-grade (zero recovery):
If a microbial filter with a 7 log (99.99999%) reduction claim were to be challenged with > 1010 (100,000,000,000) bacteria over its installation life, the expectation would be that > 1,000 bacteria would be discharged to the environment or user.
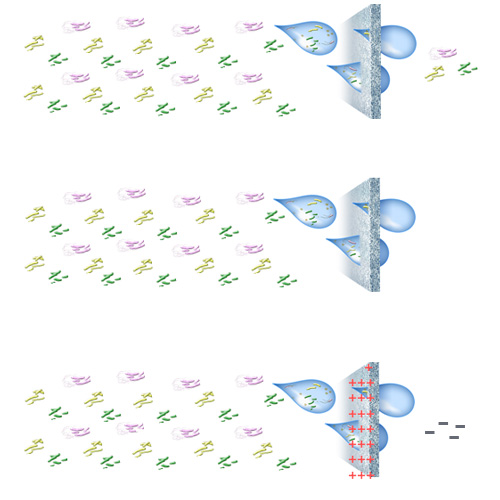
Fig 2. From top to bottom, examples of microbial, sterilizing-grade, and endotoxin filtration.
We have covered many types of physical filtration, in which particles and organisms are removed because the micron size of the filter is smaller than the particle. However, filters can also work through adsorption and chemical adsorption, such as for chemical or ion removal or for endotoxin reduction. The efficiency of these filters is often based on parameters like flow rate. If a filter like this also has a micron size rating, it is important to understand the category of the physical particle removal it falls under. This knowledge can help you to make the best decision for each filtration application.
References
- Sutherland KS. Section 1 Basic Principles. In: Filters and Filtration Handbook. Elsevier Science; 2008:1-40.
- Application note: Understanding Particle Removal Performance in Liquids with Cartridge and Bag Filtration. Pall Corporation. March 2016. https://www.pall.com/content/dam/pall/food-beverage/literature-library/non-gated/understanding-liquid-particle-removal-performance.pdf
- ASTM F838-20, Standard Test Method for Determining Bacterial Retention of Membrane Filters Utilized for Liquid Filtration. ASTM International, West Conshohocken, PA, 2020. www.astm.org.
- Food and Drug Administration (FDA), Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing – Current Good Manufacturing Practice. Docket Number: FDA-2003-D-0145. 2004 October. October. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/sterile-drug-products-produced-aseptic-processing-current-good-manufacturing-practice. [Updated 04 May 2020].
Author bio
Marissa Khoukaz - Business Development Manager— Hospital Water

Marissa is a Business Development Manager for Hospital Water and manages the portfolio globally for Cytiva. She works with high-risk units to reduce waterborne pathogen risk to patients.