Inside an IV in-line filter: learn more about IV filtration mechanisms.
The problem
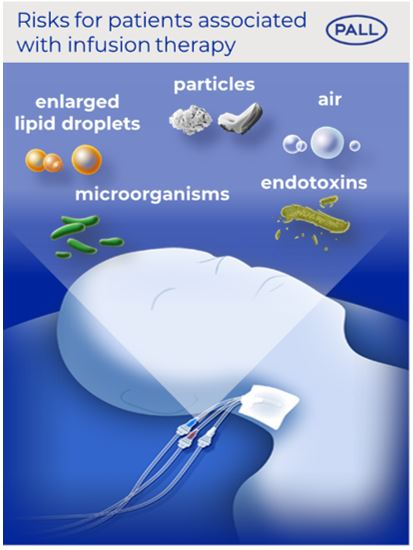
Intravenous drug infusions can be a life-saving therapy. It has been shown that these solutions may contain inadvertent particles, ambient air, microorganisms, their associated endotoxins, and enlarged lipid droplets which can potentially put the patient’s health at risk (1-4).
Our solution: Experimental and clinical studies have proven that our IV in-line filtration devices can retain particles, air bubbles, microorganisms, their associated endotoxins, and enlarged lipid droplets (5-7).
Read here about the mechanisms of IV in-line filtration and how IV in-line filter retain particles, air bubbles, microorganisms, their associated endotoxins, and enlarged lipid droplets.
Mechanisms of particles filtration
Particulates above 10 µm in solutions for intravenous therapy are regulated by European and US Pharmacopeias (4). However, smaller particles and particles inadvertently produced during preparation and administration of the solutions to the patient are not/cannot be regulated. Intensive care patients who receive a multitude of IV infusions can therefore be infused with up to one million particles per day in the absence of IV in-line filters (8-11). Infused particles could lead to a disturbance in the microcirculation and compromised microvascular flow in vital organs resulting in organ dysfunction of ICU patients (9-12).
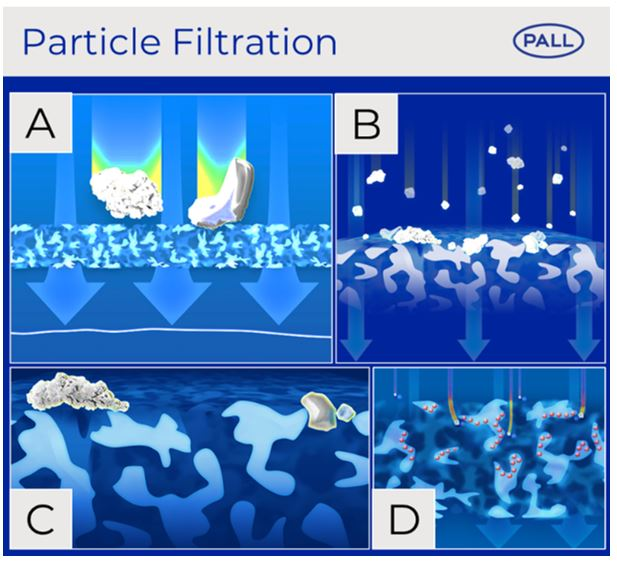
Our IV in-line filters retain particles by conventional mechanisms of direct interception which include size exclusion (A), cake filtration (B) and bridge filtration (C). Additionally, particulates may be retained by inertial impaction within the membrane or diffusion Interception where there are charge effects (D).
Mechanisms of air filtration
Estimates of the frequency of vascular air embolism (VAE) related to central venous catheters (CVCs) vary from study to study and are reported to range from 1 in 47 to 1 in 3000 catheterization events or from 0.1% to 2% per patient (13-15). While the frequency of this complication may be low, mortality rates attributed to venous air embolisms associated with CVCs range from 23% to 50% (16-20).
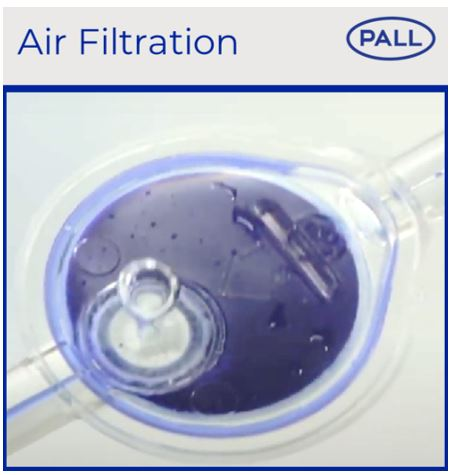
Hydrophobic vent membranes on our IV in-line filters allow effective air elimination that may be entrained in infusions (i.e., due to degassing, disconnection, etc.) and protect against air emboli in patients. Results from internal studies demonstrate that our filters can eliminate entrained air in both a vertical and horizontal position (21).
Mechanisms of microorganisms filtration
Lipid-containing parenteral nutrition (PN) solutions are often considered as an ideal microbial growth medium, and slow administration at room temperature offers the opportunity for microbes to multiply and cause adverse effects (22-24).
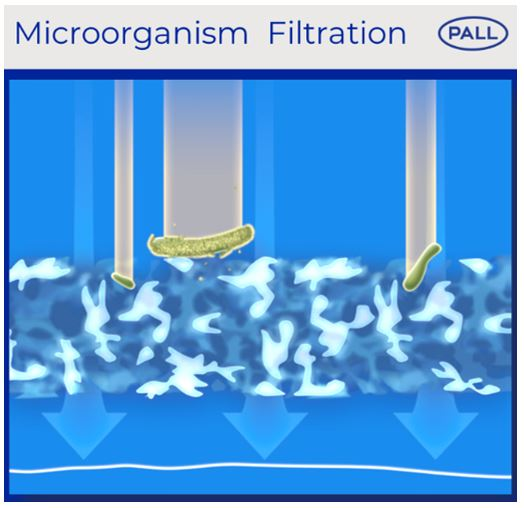
Our 1.2 µm IV filters have the possibility to retain inadvertent microbes with a size above 1.2 µm, such as Candida species. Our 0.2 µm IV filters can retain smaller inadvertent microbes, which could include Staphylococcus epidermidis and Escherichia coli.
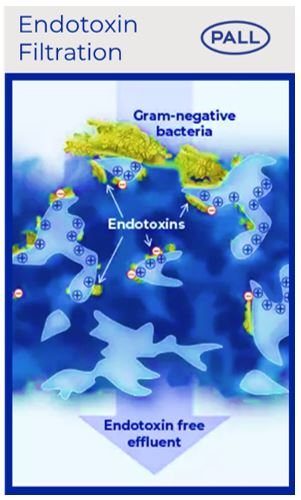
Mechanisms of endotoxin filtration
Previous studies have shown that gram-negative bacteria can multiply and will shed endotoxin in intravenous solutions (24-25). Inadvertent infusion of nonsterile fluids contaminated with endotoxins may be rare, but hospital cases of endotoxin in intravenous solutions leading to adverse reactions or even death have been investigated (26-30).
Our Posidyne™ positively charged membrane (+) retains endotoxin due to the binding to the negative charge of endotoxins (-).
Filtration of enlarged lipid droplets
Maintaining stability of lipid-containing parenteral nutrition solutions is critical, since destabilization can cause the lipid globules to coalesce, thus causing the particle size to exceed 5 µm. If particle size exceeds 5 µm patients can be at risk of pulmonary capillary occlusion and fat emboli (34).
Our 1.2 µm IV filters have the possibility to retain lipid droplets with a size above 1.2 µm.
With a strong focus on patient safety and satisfaction, our expertise on IV in-line filtration devices optimizes infusion therapy worldwide.
References
- Myers GJ. Air in intravenous lines: a need to review old opinions. Perfusion. 2017;32(6):432-435. doi.10.1177/0267659117706834.
- Merry AF, Gargiulo DA, Fry LE. What Are We Injecting with Our Drugs? Anaesth Intensive Care. 2017;45(5):539-542. doi.org/10.1177/0310057X1704500503.
- Holmes CJ, Kundsin RB, Ausman RK, Walter CW. Potential hazards associated with microbial contamination of in-line filters during intravenous therapy. J Clin Microbiol. 1980;12(6):725-731. doi:10.1128/jcm.12.6.725-731.1980.
- Perez M, Maiguy-Foinard A, Barthélémy C, Décaudin B, Odou P. Particulate Matter in Injectable Drugs: Evaluation of Risks to Patients. Pharm Technol Hosp Pharm. 2016;1(2):91-103. doi.10.1515/pthp-2016-0004.
- Perez M, Décaudin B, Abou Chahla W, Nelken B, Storme L, Masse M, et al. Effectiveness of in-Line Filters to Completely Remove Particulate Contamination During a Pediatric Multidrug Infusion Protocol. Sci Rep. 2018;8(7714):1– 8. doi.10.1038/s41598-018-25602-6.
- Van Lingen R, Baerts W, Marquering A, Ruijs GJ. The use of in-line intravenous filters in sick newborn infants. Acta Paediatr. 2004;93(5):658-662. doi.10.1111/j.1651-2227.2004/tb02993/x.
- Richards C, Grassby PF. A comparison of the endotoxin-retentive abilities of two '96-h' in-line intravenous filters. J Clin Pharm Ther. 1994;19(3):199-202. doi:10.1111/j.1365-2710.1994.tb00673.x
- Perez M, Décaudin B, Abou Chahla W, Nelken B, Barthélémy C, Lebuffe G, Odou P. In vitro analysis of overall particulate contamination exposure during multidrug IV therapy: impact of infusion sets. Pediatr Blood Cancer. 2015;62(6):1042-1047. doi:10.1002/pbc.25442.
- Benlabed M, Perez M, Gaudy R, Genay S, Lannoy D, Barthélémy C. Clinical implications of intravenous drug incompatibilities in critically ill patients. Anaesth Crit Care Pain Med. 2019;38(2):173-180. doi:10.1016/j.accpm.2018.04.003.
- Lehr HA, Brunner J, Rangoonwala R, Kirkpatrick C. Particulate matter contamination of intravenous antibiotics aggravates loss of functional capillary density in postischemic striated muscle. Am J Respir Crit Care Med. 2002;165:514-520. doi:10.1154/AJRCCM.165.4.2108033.
- Kirkpatrick CJ, Rangoonwala R, Reshetnykov M, Barbeck M, Ghanaati S. Non-Equivalence of Antibiotic Generic Drugs and Risk for Intensive Care Patients. Pharmaceut Reg Affairs. 2013;2(1):1-7. doi:10.4172/2167-7689.1000109.
- Schaefer SC, Bison PA, Rangoonwala R, Kirkpatrick JC, Lehr HA. 0.2 µm in-line filters prevent capillary obstruction by particulate contaminants of generic antibiotic preparations in postischemic muscle. Chemother J. 2008;17:172-178.
- Cook LS. Infusion-related air embolism. J Infus Nurs. 2013;36(1):26-36. doi:10.1097/NAN.0b013e318279a804.
- Boersma RS, Jie KS, Verbon A, Pampus EC van, Schouten HC. Thrombotic and infectious complications of central venous catheters in patients with hematological malignancies. Ann Oncol. 2008;19:433-442. doi:10.1093/annonc/mdm350.
- Gordy S, Rowell S. Vascular air embolism. Int J Crit Illn Inj Sci. 2013;3(1):73-76. doi:10.4103/2229-5151.109428.
- Scott WL. Complications associated with central venous catheters: A survey. Chest. 1988;94(6):1221-1224. doi:10.1378/chest.94.6.1221.
- Vesely TM. Air Embolism during Insertion of Central Venous Catheters. JVIR. 2001;12(11):1291-1295. doi.org/10.1016/S1051-0443(07)61554-1.
- Feil M. Preventing central line air embolism. Am J Nurs. 2015;115(6): 64-69. doi:10.1097/01.NAJ.0000466327.76934.a0.
- Heckmann JG, Lang CJ, Kindler K, Huk W, Erbguth FJ, Neudӧrder. Neurologic manifestations of cerebral air embolism as a complication of central venous catheterization. Crit Care Med. 2000;28(5):1621-1625. doi:10.1097/00003246-200005000-00061.
- Kashuk JL, Penn I. Air embolism after central venous catheterization. Surg Gynecol Obstet. 1984;159:249-252.
- Stephens A. Air Elimination Capabilities of the Pall Lipipor™ TNA2 Filter for Parenteral Nutrition. Pall Technical report; # 10.3992. 2010.
- Austin PD, Hand KS, Elia M. Systematic review and meta-analyses of the effect of lipid emulsion on microbial growth in parenteral nutrition. J Hosp Infect. 2016;94(4):307-319. doi:10.1016/j.jhin.2016.08.026.
- Holmes CJ, Kundsin RB, Ausman RK, Walter CW Potential hazards associated with microbial contamination of in-line filters during intravenous therapy. J Clin Microbiol. 1980;12(6):725-731. doi:10.1128/jcm.12.6.725-731.1980.
- Trautmann M, Zauser B, Wiedeck H, Buttenschӧn, Marre R. Bacterial colonization and endotoxin contamination of intravenous infusion fluids. J Hosp Infect. 1997;37(3):225-236. doi:10.1016/s0195-6701(97)90251-6.
- Jorgensen JH, Smith RF. Rapid detection of contaminated intravenous fluids using the Limulus in vitro endotoxin assay. Appl Microbiol. 1973;26(4):521-524. doi:10.1128/am.26.4.521-524.1973.
- Garrett DO, McDonald LC, Wanderley A, Miller P, Carr J, Arduino M, et al. An outbreak of neonatal deaths in Brazil associated with contaminated intravenous fluids. J Infect Dis. 2002;186(1):81-86. doi:10.1086/341083.
- Daufenbach,LZ, Alves WA, Azevedo JB de, Arduino MJ, Foster TS, Carmo EH, et al. Pyrogenic reactions and hemorrhage associated with intrinsic exposure to endotoxin-contaminated intravenous solutions. Infect Control Hosp Epidemiol. 2006;27(7):735-741. doi:10.1086/504360.
- Schroeder J, O’Neal C, Jagneaux T. Practically Saline. J Investig Med High Impact Case Rep. 2015;3(4):2324709615618980. Published 2015 Nov 27. doi:10.1177/2324709615618980.
- Endotoxin-Like Reactions Associated with Intravenous Gentamicin -- California, 1998. Department of Health and Human Services. Morbidity and Mortality Weekly Report. Published: 1998 Oct 23;47(41):877-880. Reviewed: 2001 May 02. https://www.cdc.gov/mmwr/preview/mmwrhtml/00055322.htm
- Johnstone T, Quinn E, Tobin S, Davis R, Najjar Z, Battye B, et al. Seven cases of probable endotoxin poisoning related to contaminated glutathione infusions. Epidemiol Infect. 2018;146(7):931-934. doi:10.1017/S0950268818000420
Author bio
Dr. Volker Luibl, MBA

Dr. Luibl is a Demand Generation Marketing Manager in medical device and clinical science.