Blog
Measuring Filter Performance Part 1: More than Just a Label
July 4, 2022
How do we measure a filter’s performance?
The simplest place to start is by looking on the filter or the box it was supplied in. How is it labeled? The performance, or rating, of a filter is often expressed in terms of a micron (µm) rating. This is often perceived as the smallest particle size that a filter can remove. But, while this is not untrue, it is incomplete at best, misleading and in many ways irrelevant.
Socks – an unlikely filter!
If I were to remove my socks, perhaps pack them into a toilet roll tube and pass a solution loaded with 10 µm particles through the assembly, it is highly likely that we would identify that my socks were capable of removing some 10 µm particles. We may then feel empowered to label and assemble them as a 10 µm filter. Most readers would agree that this is not a very good, or suitable filter! So, it is important we find a better way to define the performance in increasingly stringent ways to help identify the right filter for any given process.
The sock example would be an extreme case of a ‘nominal’ filter rating. This ill-defined measure of performance may be all that is required in some very basic applications and so it is important to acknowledge that it is the application that dictates the quality of the filter that needs to be used.
Beta-ratio to evaluate filter performance
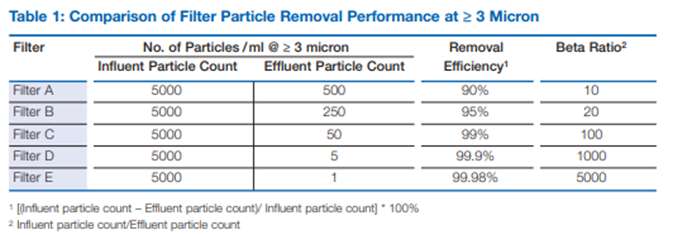
If my process were sensitive to the presence of particulate contamination above 10 µm, I should be nervous using the ‘sock’ filter. The reality is that filter performance or efficiency can be evaluated in more detail by concepts such as the beta-ratio. Simply put, this is the ratio of the incoming number of particles of a given size to the number of those particles in the effluent from the filter. A beta-ratio of 100 (β100) would equate to 99% reduction at that particle size, β1000 a 99.9% reduction and β5000 a 99.98% reduction. For most pharmaceutical grade filtration products, a β100 would be considered a very low-quality filter, only used in non-critical applications and β5000 better suited to applications where a high degree of particle control is required to maintain the critical quality attributes of the product being manufactured.
Differences in particle removal performance are highlighted in the table below.
The modified OSU-F2 test for quality release testing
In practice, a single particle rated filter may have a variety of performance figures. A common quality release test during filter manufacture is something called a modified OSU-F2 test which is based on consensus standard ISO 4572, ANSI B.93.31- 1973. This essentially challenges a filter with a test fluid containing a broad range of particle sizes, measuring the number of these in the influent and effluent under some standard conditions. This set of data allows us to establish the performance of the filter at any given micron size. This is powerful but is limited to filters with larger particle ratings (>5 µm) and assumes both non-deformable particulates and non-yielding filtration matrices that may perform differently in the presence of changing differential pressures. Neither of these assumptions are recommended to be relied upon in the most critical applications. Despite this, this test can be adequate for genuine particle-reducing applications.
Removing bacteria from a process…
If we think beyond particles and think about a myriad of bacteria to be removed from a process, even if we were able to test using this method at the sub-micron level, the performance of even a great particulate filter is orders of magnitude too low to be useful. A 99.98% (β5000) level of performance may seem like a great performing filter but when we think that bacterial contamination can easily be equivalent to millions, if not billions of particles, then this is not sufficient if we truly want to remove all bacteria from a process.
We will look at methods to qualify these filters in our next blog: Measuring Filter Performance: Is Performance Absolute?
Thank you for signing up
Thank you for signing up to the Biotech Blog.

Mark Ayles, Senior Marketing Manager
- Category
- Author
- Sort By