Traditionally, the primary formulation options for biotech drugs have been those intended for intravenous administration, due to limitations in the way that drugs intended for subcutaneous administration have been produced in the past.
However, recently the biotech industry has begun to increase the number of subcutaneously-administered biologic drugs being manufactured in order to support patient preference for self-administration over intravenously-delivered therapies. This is especially apparent with drugs at Phase II onwards (see our White Paper on High-Concentration Monoclonal Antibody Drugs: Manufacturing Challenges.
Due to the differing method of delivery, subcutaneously-adminstered drugs need to be produced at a much higher concentration level than found with intraveneously-administered drugs, and like you, we know that this presents many technical and processing challenges when working with fluids of such higher resulting viscosity.
Drug Type |
Typical Concentration |
Typical Viscosity |
|---|---|---|
IV-administered monoclonal antibody drug |
5 to 50 |
1 to 5 |
Subcutaneously-administered monoclonal antibody drug |
100 to 250 |
10 to 50 |
But while investment increases at pace in the development and manufacture of high-concentration subcutaneous monoclonal antibody drugs, are you also risking the loss of valuable product during the process due to the complex processing challenges involved?
With such an extremely high value-per-unit-volume of highly-concentrated materials, we know that no drug manufacturer or developer can afford to compromise on material losses and need to do everything possible to maximize their yields
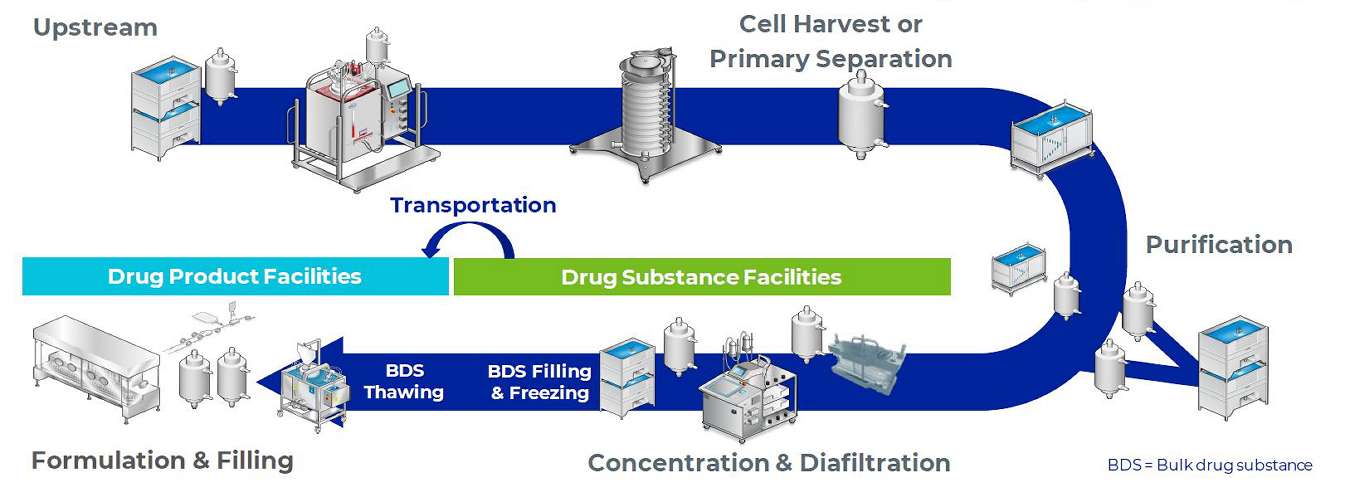
Above: a typical high-concentration monoclonal antibody drug manufacturing process
Challenges
Barriers to avoiding losses while achieving maximum yields when working with high-concentration fluids can include a manufacturer’s reliance on outdated or legacy equipment and procedures designed to produce drug concentrations typically used for intravenous delivery, resulting in oversized, inefficient unit operations.
Issue |
Impact |
|---|---|
Low filter membrane flux due to viscous fluid |
Extends processing time |
High degree of shear during TFF |
Increases risk of unwanted aggregates and diminishes ability to meet critical quality attributes, including purity |
Line losses and product hold-up due to oversizing |
Reduces yield and recovery of extremely high-value product |
Scarcity of fluid constrains process development scope |
Limits ability to optimize unit operations |
What You’ve Told Us
- You’re losing high-value material entrained in sterilizing filters in closed systems
- You’ve underestimated the impact that hold-up volume has on yields as you scaled up the process
- Viscous products stick to tubing and bioprocess containers, and can be a pain to recover
- Filters with insufficient capacity for viscous, high concentration products are detrimental to yield
- You need to perform serial concentration steps to achieve your target concentration
- It can be much harder to achieve uniform mixing and you risk creating doses of drug product that are out of specification
- With high-concentration drugs you employ a more stringent target for aggregates pre-freeze and thaw
- Clogging of needles during aseptic filling creates downtime and reduces yields
- Your existing pumps don’t have the capacity for viscous, high-concentration fluids
- Your final ultrafiltration (UF)/diafiltration (DF) step creates particulates in the bulk drug that tend to block your sterilizing-filter
How We Can Help You
Tom Watson, Group Leader, Product Management for Direct Flow Filtration and Formulation and Filling
- Do you use the same technologies and products to produce drugs with increased drug concentrations that you use for low or standard-concentration drugs?
- Are you experiencing any process challenges when working with higher concentrations of drug substance?
- Which workarounds, if any, do you perform to overcome these challenges?
It’s clear from your past feedback and the research we’ve undertaken that there are many difficult, technical issues to overcome when dealing with high-viscosity drugs. However, our team is perfectly-positioned to support and help you navigate the challenges of developing your high-concentration monoclonal antibody drugs - with the right products and support, especially process development and validation services where and when you need it.
We will deliver a platform of solutions at every process step: from concentration and concentration monitoring; bulk drug substance filtration, dispensing and storage; filling, freezing, transporting, and thawing that can be individually tailored toward your own high-concentration drug development and manufacturing objectives.
We also have the advantage very strong technical knowledge and experience in our Scientific and Laboratory Services team with a wide understanding of your typical processes and challenges.
For advice, help, and direct support for all of your high-concentration monoclonal antibody drug processing challenges.
References
Wiley Online Library. “Strategies for high-concentration drug substance manufacturing to facilitate subcutaneous administration: A review”. Biotechnology and Bioengineering. July 2020.
https://onlinelibrary.wiley.com/doi/10.1002/bit.27510
ScienceDirect. “Ongoing Challenges to Develop High Concentration Monoclonal Antibody-based Formulations for Subcutaneous Administration: Quo Vadis?”. Journal of Pharmaceutical Sciences. April 2022. https://www.sciencedirect.com/science/article/pii/S0022354921006146
PubMed.gov. “Filling of High-Concentration Monoclonal Antibody Formulations into Pre-filled Syringes: Investigating Formulation-Nozzle Interactions To Minimize Nozzle Clogging”. PDA Journal of Pharmaceutical Science and Technology. June 2015.
https://pubmed.ncbi.nlm.nih.gov/26048747/
World Health Organization. “Call for consultant on monoclonal antibodies for infectious diseases”. July 2021. https://www.who.int/news-room/articles-detail/call-for-consultant-on-monoclonal-antibodies-for-infectious-diseases
Biopharm Services Ltd. “Antibody Drug Conjugates aren’t “too expensive” –how can the costs of manufacture be monitored and improved?”. January 2021.
IAVI. “Expanding access to monoclonal antibody-based products” https://www.iavi.org/phocadownload/expanding/Expanding%20access%20to%20monoclonal%20antibody-based%20products.pdf
PharmTech. “Considerations for Sterile Filtration of Biologic Drugs”. Pharmaceutical Technology. July 2019. https://www.pharmtech.com/view/considerations-sterile-filtration-biologic-drugs
Drug Discovery & Development. “High Concentration Biologic Formulations: Challenges and Solutions” June 2017. https://www.drugdiscoverytrends.com/high-concentration-biologic-formulations-challenges-and-solutions/
PubMed.gov. “US FDA-approved therapeutic antibodies with high-concentration formulation: summaries and perspectives”. Antibody Therapeutics. October 2021.
https://pubmed.ncbi.nlm.nih.gov/34909579/