VacuCap™ Vacuum Filtration Device
Small volume media filtration up to 5 L, ideal for filtering bulk media into several containers.View Product

Acrodisc® Sterile Syringe Filters
Feed, nutrients, antibody and small media additions up to 150 mLView Product

Acro® Vent/In-line Filters
CO2 gas filtration to keep cultures sterile, protect from environmental contamination and protect vacuum pumps.View Product

AcroPrep™ 24-well Sterile Filtration Plate
Sterile filter media, reagent, and proteins in a 24-well plate formatView Product
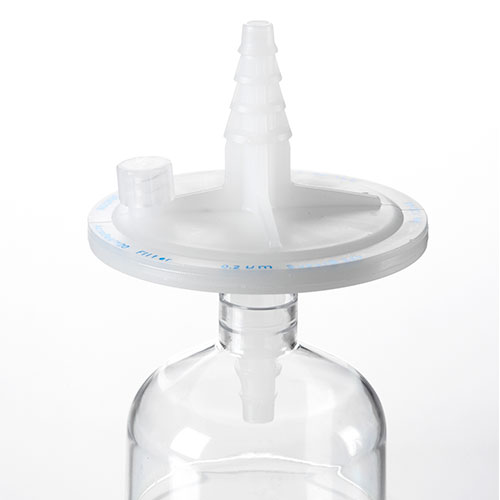
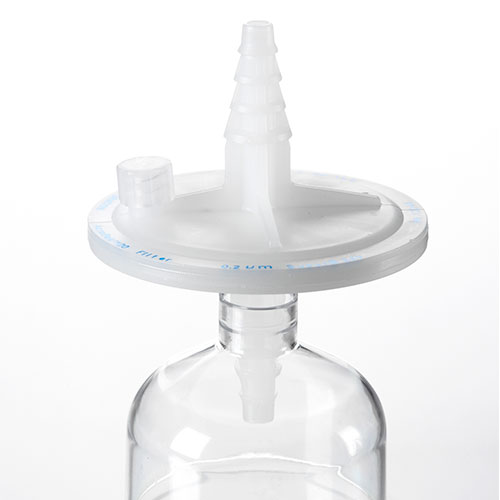
AcroPak™ 20 PES (Scale up) 50mm Filters
Sterile media filtration up to 2 L in a scale-up to GMP deviceView Product
AcroPak™ 20 PVDF 50mm Filters & 200 PVDF Capsule Filters
Sterile media filtration up to 20 LView Product


AcroPak™ 200 PES (Scale up) Capsule Filters
Sterile media filtration up to 20 L in a scale-up to GMP deviceView Product