Industry Interest Groups
1 What is BPOG?
The BioPhorum Operations Group, abbreviated as BPOG, is a global cross industry collaboration between developers, suppliers, and manufacturers to to the biopharmaceutical industry. Collaborative efforts are focusing on driving biotech industry improvements to enable the production of safer and more cost-effective drugs. Collaboration areas are divided into Phorums, and Pall Biotech is a member of two: Technology Roadmapping (TR) and Supply Partner (SP) phorums. Actively participating in these groups are representatives from across all areas of the business including Pall Biotech’s quality, supply chain, R & D, and engineering teams. Active participants collaborate to drive technological advancement and continuous improvements in business.
2 Pall Biotech’s Activity in BPOG
2.1 Technology Roadmapping Phorum
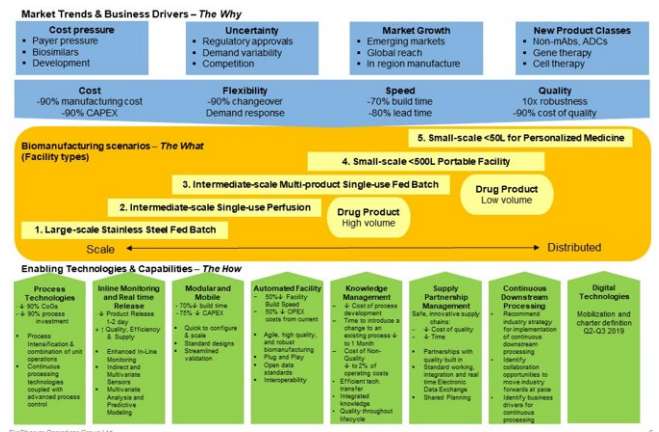
The primary objective of the Technology Roadmapping (TR) phorum is the establishment of a process, adopted by industry, which will accelerate change by overcoming common technological challenges and developing solutions that benefit all stakeholders. The current technology management process, see Figure 1 below, accelerates innovation, development of the manufacturing processes of the future, and the availability of more affordable lifesaving drugs.
Figure 1. BPOG technology roadmap vision (Courtesy of BPOG)
Current works include:
- Proving through concept demonstrators plug and play hardware
- Designing a buffer skid to reduce buffer preparation footprints
- Outlining the technology and regulatory barriers in the continuous downstream processing of therapeutic proteins - driving the move for more standard facility design to accelerate capacity builds.
2.2 Supply Partner Phorum
In the Supply Partner (SP) phorum, suppliers and drug manufacturers are working collaboratively to build the modern and effective inbound supply chains that our industry needs. Three programs have been recently prioritized:
- Pilot for a transformative joint audit program, with the aim to improve the quality of audits for single-use systems (SUS) while decreasing the burden both on the supplier and end user side;
- Aggregated Biopharma capacity analysis to forecast future demand resulting from the current large manufacturing capacity build;
- Risks and business continuity planning.
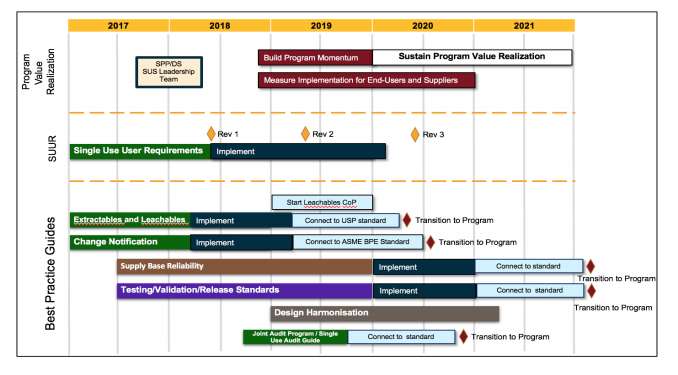
In addition, recognizing the importance of single-use technologies (SUT) in current biomanufacturing, a disposable five-year plan (Figure 2) has been designed to enable single-use components, assemblies, and technologies to be utilized with the same level of confidence traditionally conferred to stainless steel.
Figure 2: BPOG disposable five-year plan (Courtesy of BPOG
Notable among the achievements that have already resulted from this plan, we want to mention:
- “An Industry Proposal for Change Notification Practices for Single-Use Biomanufacturing Systems.” A white paper describing a harmonized risk-based approach to single-use change notifications, thus lowering risk to licensees by defining appropriate notification periods with the supply base.
- The Single-Use Users Requirements (SUURS): was developed as an industry-wide harmonization approach between suppliers and end users by creating a consistent and complete technical package. This package includes a quality audit guide, internal/external quality release procedure, and a template for user requirements.
- A recommended extractables testing protocol that provides suppliers with a set of agreed upon procedures as representing a comprehensive range of conditions by a broad group of companies. This also includes templates for Extractables Test Reports. It is anticipated that end users can therefore accelerate their risk assessments pertaining to single-use components.
3 Conclusion
Pall Biotech is heavily involved in these BPOG industry group activities and is committed to implement these best practises. As an example of our willingness to move the industry forward, for many years we have committed extensive resources to the execution of extractable testing on our single-use components according to the BPOG recommendations.
Doc ref: RPSCIG_03