Bio-Process Systems Alliance Recommendations
Current ways to assess and demonstrate proper assurance of integrity of SUS are based on risk assessment. The Bio-Process Systems Alliance (BPSA) developed a recommended approach in the technical guide: Design, Control, and Monitoring of Single-Use Systems for Integrity Assurance (July 2017).
Many suppliers and end-users of SUS follow this approach to define their strategy for assurance of integrity of SUS and their associated practices.
This risk management approach considers:
- The intended application of the SUS and its criticality in terms of patient safety, drug safety, operator safety, and/or business impact.
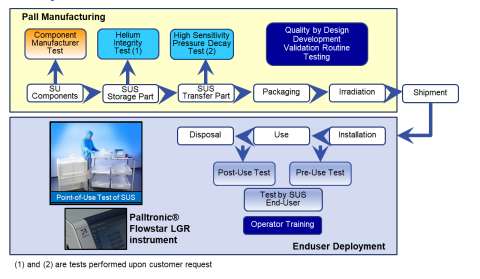
- The whole life cycle of the SUS, from its design and manufacturing at the supplier’s site to its disposal at the drug manufacturer’s site (see Figure 1)
- The supplier qualification and manufacturing practices:
- Quality by Design (QbD) approach, validation/verification tests (like junction tests), etc
- Packaging and transportation validation tests
- Operators' training
- Routine testing and controls (visual inspection, leak/integrity tests, etc.)
- The drug manufacturer qualification and manufacturing practices:
- Verification of SUS attributes against the requirements for the intended use, also referred to as the User Requirement Specifications (URS)
- Procedures for storage, unpacking, installation and use of the SUS:
- Visual inspection o Operator training
- Leak/integrity tests
In most instances, the standard practices of the SUS supplier are sufficient to ensure the required level of assurance of integrity for the SUS application. However, when dealing with sterile substance storage or aseptic processing, there might be a need for specific additional tests. Assuring the integrity of a SUS is a shared responsibility between the supplier and the drug manufacturer. It requires a strong collaboration and continued relationship, as well as a full understanding of the SUS application's requirements.
Figure 1. Typical life cycle of a Pall Biotech SUS
Additional Resources
- Bio-Process Systems Alliance (BPSA)
- Technical guide: Design, Control, and Monitoring of Single-Use Systems for Integrity Assurance (July 2017) – http://bpsalliance.org/technical-guides
- Article : Recommended Practices for Assuring Integrity of Single-Use Systems - https://bioprocessintl.com/manufacturing/single-use/recommended-practices-for-assuring-integrity-ofsingle-use-systems/
- ASTM E55.04 (work in progress)
- WK64337 Best Practices - Integrity Assurance and Testing of Single-Use Systems
- WK64975 Testing Method – Microbial Ingress Testing on Single-Use Systems
- USP<1207> Sterile Product Packaging - Integrity Evaluation (May 2018)
- Webinar: Ensure integrity of single-use systems for vaccines sterile processing, Charlotte MASY, Technical Lifecycle Project Manager SUS, TLCM-MSAT GSK Vaccines and Nathalie PATHIER, Senior Marketing Manager, Pall Biotech - https://biotech.pall.com/en/webinars/2018-webinar-ensure-integritysingle-use-systems-vaccines-sterile-processing.html?_ga=2.154710136.1133267518.1546442294- 85224273.1543311764 BioPhorum Operations Group (BPOG) and Bio-Process Systems Alliance (BPSA) Single Use User Requirements (SUUR) toolkit - https://www.biophorum.com/category/resources/suur/about-suur/
Doc ref: RPSCIN_02