Explore Biotech Products, Systems and Solutions
From concept through to design, biotech validation and production across all biotech applications, we can help you adapt to changing market conditions
with innovative technologies and solutions. Whether that means quickly developing a process for a new drug, vaccine production, helping you ensure regulatory compliance,
or making existing processes easier, faster and better, we are ready to work with you to address your process needs.
Gene Therapy and Oncolytic Viruses
Overcome development and viral vector manufacturing obstacles to accelerate delivery of commercially viable gene therapies to patients.
Monoclonal Antibodies and Recombinant Proteins
A portfolio to support all your monoclonal antibody production choices, including traditional manufacturing methods and single-use alternatives.
Vaccine Production
Enhanced solutions to meet the most difficult vaccine development and manufacturing challenges, and your specific vaccine production needs.
Contact Us
Dedicated support to help address your process challenges and enable you to improve your speed to market.
Blog Post
Each different process comes with its own unique challenges, perhaps none more so than viral vectors.
In this blog, Paul Cashen takes an in-depth look at different adeno-associated virus (AAV) production process scale-up strategies.
On-Demand Webinar
What should you consider when building an integrity control strategy for your single-use systems (SUS)? How do you overcome application challenges? Join leading industry expert Charlotte Masy of GSK and to gain insights and answers.
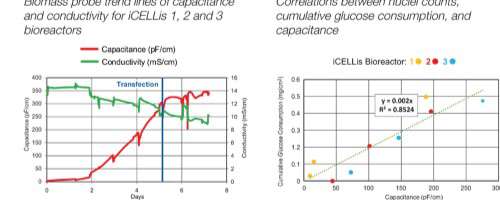
Poster
Todd Lundeen and Andrew Laskowski take a detailed, technical look at how Aber’s FUTURA biomass proble provides reliable measurements when used with the iCELLis® nano bioreactor, correlating cumulative glucose consumption, nuclei counts and capacitance.
More Posters and Presentations
Single-Use Platform for Scalable Purification of a VSV-G Lentiviral Vector
View All Posters and Presentations »
Trends in Single-Use Mixing Technologies
It is known that the current market drivers in the biotech industry demand process innovation to increase manufacturing flexibility and reduce production costs. This results in increased pressure on bioproduction platforms to become more versatile whilst maintaining high productivity and minimal down time.
Mixing equipment needs to adapt to these requirements by becoming interchangeable and more scalable in order to handle wider ranges of volumes and more varied applications, without loss in efficiency.
Read our case study on Lonza's Ibex® Solutions to learn more on the current mixing technology landscape, and gain insight on a modular approach to mixing equipment.
All the latest news and offers direct to your inbox
Sign up today to get the latest industry insights emailed to you, plus news, trends, early notice of new webinars and virtual events, special offers, invitations, discount codes and promotions. We'll also keep you informed of many groundbreaking new technologies, solutions and products.
Integrated Solutions
Moving to integrated solutions will help simplify and streamline your manufacturing process, speeding time to market and reducing overall cost of goods. The simplified process using standard equipment, qualified designs and components lowers the risk for operator error, which helps assure compliance with cGMP and assure product quality. Our integrated solutions team combines best engineering and project management practices with in-depth industry knowledge and diverse equipment portfolio to deliver these benefits on time, and on budget.
Accelerator℠ Documentation Center
Transparency and partnership are at our heart. We know the availability of high quality product documentation helps manage the documentation required for regulatory submission.
The Accelerator Documentation Center collates all current documentation to help you navigate compliance quickly and efficiently, and the electronic regulatory dossiers help you in your ongoing risk assessments to help alleviate the pressures of preparing documentation for regulatory compliance and audits.