Haga clic en el siguiente enlace para restablecer su contraseña.
Si no logra solucionarlo, póngase en contacto con nosotros
En general, se debe seleccionar un MWCO de 3 a 6 veces más pequeño que el peso molecular de la proteína u otra especie que deba retenerse. Si la velocidad de flujo o el tiempo de procesamiento es una consideración importante, se debe elegir una membrana con un MWCO en el extremo inferior de este rango (3 veces); si la retención es el objetivo principal, se debe elegir una membrana más cerrada (6 veces). Estos valores deben considerarse una guía, puesto que la retención y la selectividad de solutos pueden variar según muchos factores, como la presión, la forma molecular, la presencia de otros solutos y las condiciones iónicas. Debido a su forma compleja, las proteínas se comportan de manera diferente de los ácidos nucleicos, que son lineales y flexibles. Para estos últimos, la adecuación del MWCO del dispositivo centrífugo está correlacionada con el rango de longitud de fragmentos. Puesto que puede forzarse a las moléculas de ácido nucleico a pasar por membranas de muchos MWCO independientemente de su tamaño, una retención efectiva de una membrana de ultrafiltración requerirá una reducción en la fuerza G. Para la retención máxima del ácido nucleico, las velocidades de los dispositivos Nanosep no deben superar 5000 x g y se recomienda una velocidad en el extremo inferior del rango de fuerza G para los demás dispositivos centrífugos.
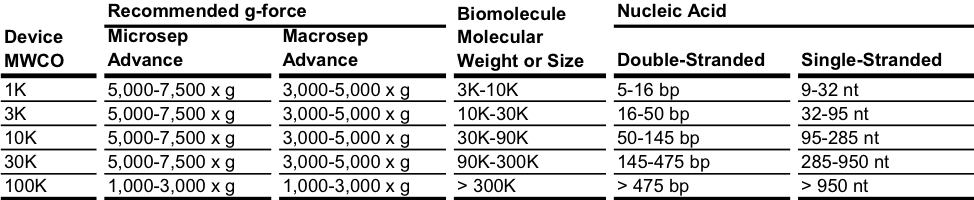
La membrana 100K tiene un tamaño de poro nominal de 10 nm y es apta para biomoléculas/partículas superiores a los 30 nm. Se recomienda realizar el centrifugado con ácidos nucleicos en el extremo más bajo del rango de fuerza centrífuga
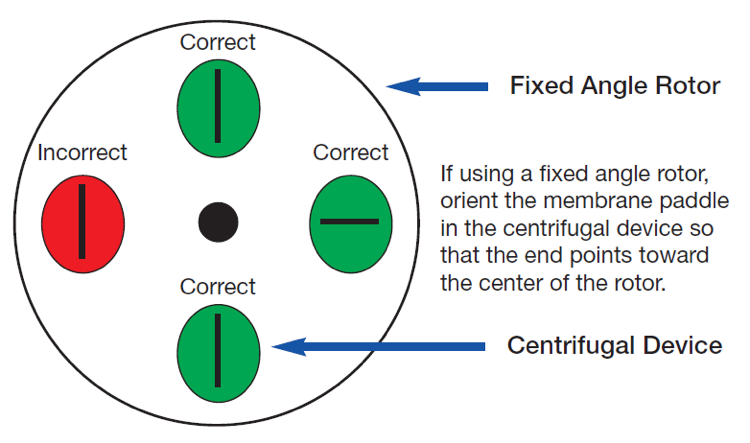
Si se usa un rotor de ángulo fijo, la paleta de membrana en el depósito de muestras del dispositivo centrífugo debe estar en el eje radial del rotor, como se indica en el siguiente esquema. Aunque la orientación incorrecta no afecta la integridad ni la retención de la membrana, aumenta el tiempo de procesamiento y afecta el factor de concentración de muestra final que se puede lograr.
Pall ofrece cuatro dispositivos centrífugos distintos diseñados para procesar muestras de volumen cada vez mayor. El dispositivo Nanosep® está diseñado para filtrar muestras con volúmenes de hasta 500 µL; el dispositivo Microsep™ Advance es para muestras de hasta 5 mL; el dispositivo Macrosep® Advance es para muestras de hasta 20 mL; y el dispositivo Jumbosep™ es para muestras de hasta 60 mL. La carcasa del dispositivo Jumbosep es reutilizable y solo se deben cambiar los accesorios de membrana entre un experimento y el siguiente. Los otros tres dispositivos están diseñados para uso único.
Para encontrar estos productos, visite Dispositivos centrífugos
No, los dispositivos no son estériles. Los dispositivos pueden desinfectarse usándolos para filtrar etanol al 70 % antes del uso.
Determinadas proteínas, especialmente cuando se diluyen, suelen mostrar una alta afinidad respecto de diversas superficies, lo que puede generar uniones irreversibles. Aunque los dispositivos centrífugos de Pall están fabricados con materiales con una baja afinidad intrínseca para las proteínas, este fenómeno puede causar una recuperación inferior. La recuperación de proteínas puede mejorarse a través de la “pasivación” del dispositivo. Se trata de un tratamiento previo que tiene el objetivo de ocupar los posibles sitios de unión filtrando una solución con proteínas adicionales (con frecuencia albúmina), detergentes o sales a través del dispositivo centrífugo antes del uso.
La muestra ocupa un canal contiguo perpendicular a la paleta de membrana de filtro en el fondo del depósito de muestras y por lo tanto no es necesario aspirar el material retenido de ambos lados de la paleta de membrana de filtro. Simplemente introduzca la pipeta en el canal y extraiga el material retenido.
La azida sódica agregada se agrega a la membrana de ultrafiltración de polietersulfona modificada Omega™ como un preservativo bacteriostático. Puesto que la azida sódica inhibe la oxidasa citocromática en la cadena de transporte de electrones mitocóndrica y puede inducir apoptosis, su presencia en el material filtrado puede tener efectos no deseados en las células vivas o en los estudios in vivo. La azida sólida también puede interferir con las reacciones de conjugación dependientes del grupo de aminos. Si se anticipan estos problemas u otros similares, puede realizarse un paso de enjuague previo a fin de remover la azida sódica de la membrana antes de filtrar las muestras. Se debe tener en cuenta que el dispositivo debería usarse antes de transcurridos 20 minutos de completar el paso de enjuague precio a fin de prevenir daños irreversibles en las membranas debido a la deshidratación.
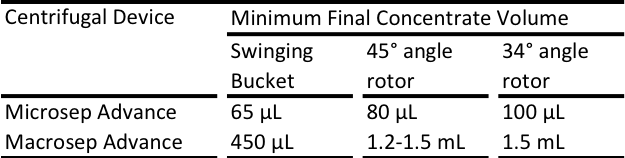
El volumen total de líquido en los dispositivos centrífugos Microsep Advance y Nanosep determinan el volumen final del material retenido. Al agregar solución amortiguadora debajo del accesorio del dispositivo, puede establecer el volumen de parada final y, por lo tanto, seleccionar el factor de concentración. Este enlace le permitirá descargar la Guía de selección de concentración para dispositivos centrífugos Nanosep y Microsep Advance, que contiene toda la información sobre cómo hacer esto.
Los colores reflejan el MWCO de la membrana Omega
Amarillo – 1K
Gris - 3K
Azul - 10K
Rojo - 30K
Translúcido - 100K
Anaranjado - 300K
El paso de enjuague previo para remover los rastros de glicerina y azida sólida presentes en la membrana de ultrafiltración de polietersulfona modificada Omega™ y desinfectar los dispositivos debe llevarse a cabo antes de la desinfección del dispositivo centrífugos.
El tratamiento ultravioleta de dispositivos Microsep y Macrosep no se ha probado. La degradación de las membranas y la pérdida de integridad podrían ser motivo de inquietud. Se recomienda la desinfección mediante el filtrado de etanol al 70 % por los dispositivos antes del uso.
El material retenido puede recuperarse fácilmente inclinando el depósito de muestras hacia el costado y extrayendo el líquido con una pipeta (puede ser necesario utilizar una punta de pipeta de 1 ml para recuperar el material de los dispositivos centrífugos Macrosep® Advance). Retirar el depósito de muestras del tubo principal facilita la tarea de ver/retirar la muestra.
No se han probado los dispositivos Microsep y Macrosep para determinar su compatibilidad con la irradiación gamma. Los dispositivos pueden desinfectarse usándolos para filtrar etanol al 70 % antes del uso.
Pall cuenta con un robusto sistema para gestionar la continuidad comercial y una sólida infraestructura global y local que permiten minimizar las interrupciones en nuestros negocios. Además, Pall implementó un plan integral para responder a la pandemia de COVID-19 que permite minimizar el impacto en nuestros clientes sin dejar de lado la seguridad de nuestros asociados y las comunidades.
Pall prioriza la salud y el bienestar de los asociados para que puedan trabajar sin correr riesgos durante la fabricación de productos y la prestación de servicios esenciales. Tomamos medidas para evitar la propagación de la COVID-19 en nuestras instalaciones e incentivar el distanciamiento social.
Las soluciones de Pall son muy importantes para la lucha contra la COVID-19, desde filtros respiratorios para respiradores hasta filtros de captura de partículas de alta eficiencia para mejorar la calidad del aire en cabinas de aeronaves y procesos para agilizar la fabricación de vacunas. Las soluciones de Pall también se usan a nivel mundial en investigaciones sobre la enfermedad.
El plato de filtro de 24 receptáculos AcroPrep se puede usar en dispositivos centrífugos equipados con un rotor de recipiente giratorio compatible. La fuerza centrífuga recomendada es de 1000 × g.
Nota importante: La placa de filtro viene con su placa de recepción exclusiva y, cuando los coloca uno encima del otro, tienen una altura combinada de 7,5 cm (2,97 in). Para operación sin problemas con rotores de recipiente giratorio en el dispositivo centrífugo, es fundamental verificar que haya espacio suficiente en el rotor para permitir un movimiento giratorio libre del transportador de microplaca con la placa de filtro apilada.
La placa de filtro de 24 receptáculos AcroPrep se ha diseñado para cumplir con los estándares ANSI/SLAS X-2004 para microplacas, lo que significa que ocupa un espacio de 12,8 cm (5,0 in) de largo y 8,6 cm (3,4 in) de ancho. La placa de filtro tiene una altura de 3,9 cm (1,5 in). La placa de filtro viene con su placa de recepción dedicada, que tiene las mismas dimensiones. Cuando la placa de recepción y la placa de filtro están apiladas, tienen una altura combinada de 7,5 cm (2,97 in).
Nota: Esta altura de placa es una consideración importante para el uso con rotores de recipiente giratorio en el dispositivo centrífugo. Es fundamental verificar que haya espacio suficiente en el rotor para permitir un movimiento giratorio libre de los transportadores de microplaca con la placa de filtro apilada, para permitir una operación sin problemas del dispositivo centrífugo.
Sí, tanto la placa de filtración estéril y clarificación celular de 24 receptáculos AcroPrep como la placa de filtración estéril de 24 receptáculos AcroPrep son dispositivos embalados individualmente y sometidos a irradiación gamma, que proporcionan una filtración de grado estéril. Las placas proporcionan un efluente estéril después del desafío de líquido con Brevundimonas diminuta en condiciones de laboratorio a un nivel de desafío de ≥107 cfu/cm².La placa de filtración estéril y clarificación celular de 24 receptáculos AcroPrep combina clarificación celular y filtración estéril en un solo paso del flujo de trabajo.
La placa de filtro de 24 receptáculos AcroPrep puede usarse para el procesamiento de vacío. El colector de vacío de placa con múltiples receptáculos de Pall (número de pieza 5017) es apto para esta aplicación. El vacío operativo recomendado es de ≥ 25,4 cmHg (10 inHg). En experimentos con células de mamíferos cultivadas (CHO y HEK293T), se determinó que un vacío de 38,1 cmHg (15 inHg) funciona bien.
El volumen de retención de una placa de filtro es el volumen de la muestra retenida por la placa y no puede recuperarse después de la filtración. El volumen de retención típico de la placa de filtración estéril y clarificación celular de 24 receptáculos AcroPrep se especifica como < 0,4 mL/receptáculo.
Durante el desarrollo, la placa de filtración estéril y clarificación celular de 24 receptáculos AcroPrep se ha usado con éxito para la clarificación de cultivos celulares de 5 mL con densidades de > 25 x 106 células/mL a una viabilidad de 90 % (CHO y HEK293T).
La placa de filtro de 24 receptáculos AcroPrep tiene un volumen de receptáculo de 7 mL. Toda esta capacidad está disponible para el procesamiento mediante vacío, en el que el volumen de trabajo recomendado es de 7 mL. No obstante, para el centrifugado, el uso de un rotor de recipiente giratorio limita el volumen de trabajo recomendado a 6 mL.
El volumen de retención de una placa de filtro es el volumen de la muestra retenida por la placa y no puede recuperarse después de la filtración. El volumen de retención típico de la placa de filtración estéril de 24 receptáculos AcroPrep se especifica como < 0,2 mL/receptáculo.
Pall participa en un consorcio a cargo del Jenner Institute, Universidad de Oxford (Oxford, Inglaterra), para agilizar y aumentar proporcionalmente el desarrollo y la producción de la vacuna contra la enfermedad. Como parte del consorcio, Pall desarrollará un proceso de fabricación a gran escala para producir millones de dosis de la vacuna. Aquí encontrará más información: Pall Corporation participa en un consorcio para desarrollar rápidamente una vacuna contra la COVID-19.
Los filtros de cada receptáculo de la placa de filtro tienen un EFA de 1,57 cm² (0,243 in²).
Los dispositivos centrífugos Nanosep® no tienen un volumen de parada final, pero la membrana siempre retiene algo de humedad. Por lo tanto, aunque parezca que el dispositivo ha girado hasta quedar seco, en la mayoría de los casos puede recuperarse el material retenido utilizando una pipeta para extraer un volumen pequeño de solución amortiguadora sobre la superficie de la membrana. No obstante, es posible establecer un volumen de parada final colocando solución amortiguadora debajo del accesorio. Consulte la guía de concentración de Nanosep®. Los dispositivos centrífugos Microsep™ Advance y Macrosep® Advance sí tienen volúmenes de parada final integrados.
No, la interferencia no es un problema con las placas de filtro de 24 receptáculos AcroPrep cuando las muestras se procesan según se recomienda.
Con las placas de filtro, se usa el término “interferencia” cuando el material filtrado de un receptáculo determinado se encuentra en receptáculos adyacentes. En principio, la interferencia puede producirse antes de la filtración debido a la exudación de la muestra de uno de los receptáculos de la placa de filtro a otro, o durante la filtración debido a la deposición del material filtrado en receptáculos adyacentes de la placa de recepción.
Puesto que los receptáculos de muestra de las placas de filtro de 24 receptáculos AcroPrep son completamente independientes, no hay posibilidades de que una muestra pase de un receptáculo a otro antes de la filtración. Esto, combinado con las mayores distancias de punta a punta de los conectores de la placa de filtro de 24 receptáculos en comparación con las que se encuentran en las placas de filtro tradicionales de 96 o 384 receptáculos, significa que la interferencia no debe ser motivo de preocupación si se respetan los volúmenes de procesamiento máximos.
Las bolsas de filtro BOS de Pall se fabrican completamente sin uniones. La composición de material única de las bolsas de filtro sin uniones Polymicro® proporciona una alta eficiencia con distribución de tamaño de poros graduada y facilita la filtración absoluta.
The Forward Flow Test is designed to test the integrity of sterilizing and virus grade hydrophilic and hydrophobic membrane filters. This qualitative test is based on measuring the gas flow across a completely wetted membrane at a defined constant test pressure on the upstream side. When the downstream side of the membrane is at atmospheric pressure a diffusion flow of gas is established due to the pressure differential. The Forward Flow test is Pall’s recommended integrity test for capsule and cartridge filters.
Yes, the SUTFF module requires a SUTFF module holder. The SUTFF module must be placed in the SUTFF module holder and compressed to activate the seals. The SUTFF module will leak if it is used without a module holder. Refer to FAQ: What is the part number of the SUTFF module holder?
Measured water flow that exceeds the test limit during Water Intrusion testing can be caused by a range of root causes, some of which are listed below. It can be seen that there are several factors which can lead to false test failures.
Possible root causes for false failures are:
- System leaks (filter housing, fittings, tubes, etc.)
- Insufficient test time
- Insufficient stabilization time
- Temperature influence
- Reversible partial wetting of the filter membrane (due to condensation of moisture within the pores or excessive pressure events)
More unlikely for unused filters (pre use test):
- Foreign substances / contaminations deposited on the filter
Root causes for true failures are:
- Filter defects
- Compromised O-ring seal with the housing
The WIT is performed on a non-wetted (“dry”) hydrophobic filter. The upstream side of the filter assembly is completely filled with water, covering the entire filter. An air test pressure lower than the actual water intrusion (or water breakthrough) pressure of the largest membranes pores is applied to the system. The membrane pores remain “dry” during the test. The test pressure drives a transport of water vapors from the water phase across the filter membrane following the pressure differential. Transport of liquid water through all wetted pores and wetted flow pathways or defects will also occur. The WIT quantitatively measures the sum of water vapor (evaporation) and liquid water flow through the hydrophobic filter.
For a sterilizing grade filter, the maximum allowed flow (integrity test limit) is derived from the generic filter validation and correlated with the bacteria retention capability of the filter.
For more information, please see Pall Publication, USD 3033 Application Note: Best Practices for Successful Filter Integrity Testing Using the Water Intrusion Test (WIT) Method
Pall recommends the use of water to lubricate the O-rings of a filter in order to ease installation into the housing.
Pall does not recommend the use of alcohol (or an alcohol/water mixture) as it could come into contact with the filter membrane, causing a hydrophilic spot that will allow water passage, resulting in a false failure test result, especially in a water intrusion test.
Additionally, if any alcohol gets trapped between the two O-rings grooves, there will be a localized area where the alcohol will expand when the filter is sterilized (autoclave or steam-in-place). This can potentially damage the filter adapter, or impact the filter to housing seal.
For more information, please see Pall Publication, USD 3033 Application Note: Best Practices for Successful Filter Integrity Testing Using the Water Intrusion Test (WIT) Method
In general, Pall does not recommend performing an integrity test at temperatures above 50°C when using water as the wetting fluid.
Forward Flow measurements at temperatures above 50 °C are considerably higher than at ambient temperature, and are more difficult to keep constant to the ±1 °C test specification. This may introduce inaccuracies in the measurement and in calculating appropriate test limits. When integrity testing is performed at elevated temperatures, the use of jacketed housing or a heating jacket is recommended to keep the temperature stable during the test.
While Pall can provide calculated test limits for elevated temperatures when water is used as the wetting fluid, additional testing should be performed to confirm these limits. In addition, monitoring actual test results by the end user can also show that these limits are appropriate.
Please contact a Pall representative if you would like more information on developing Forward Flow and Bubble Point limits for all wetting fluids at elevated temperatures.
The Bubble Point Test is designed to detect the largest pores of hydrophilic and hydrophobic membrane filters. The Bubble Point test is based on measuring the gas flow across a completely wetted membrane at increasing gas test pressure, until the point at which the wetting fluid is expelled from the pores, and bulk flow is measured.
The Bubble Point test is considered a subjective test, and the results can vary depending on the algorithm of the test instrument. The Bubble Point method is the preferred integrity test method for testing filter discs, as the Forward Flow is often too low to be accurately measured.
The Water Intrusion Test measures water flow through a submerged filter when pressure is applied to the upstream side of the filter housing. Because this test can only be performed on a hydrophobic filter, the WIT measurement for an integral filter is primarily evaporative flow of water through the pores of the membrane.
When a hydrophobic filter becomes partially wetted with a low surface tension liquid such as an alcohol water mix, or condensate (from autoclave or Steam-In-Place (SIP)), then the WIT may result in a false failure. This is due to a water channel forming through the membrane in areas where it has become wet, resulting in the free flow of water.
If the filter has become partially wet, it must be restored to a fully dry state before a WIT can be performed successfully. Flowing compressed air through the filter for several hours is often required. Alternatively, oven drying can be performed. Please contact your local Pall representative for the appropriate filter drying conditions.
Pall recommends the following to prevent a hydrophobic filter from becoming wet:
- Keep the filter away from potential sources of low surface tension liquids such as alcohol mixtures.
- If the filter is autoclaved, use a slow exhaust cycle and a vacuum drying cycle.
- If the filter is subjected to SIP, use a cooling gas such as air or nitrogen following SIP.
In certain applications, post-use testing using WIT is impractical due to product contamination on the filter, i.e. bioreactor exhaust filter. In these cases, Pall recommends performing a post-use Forward Flow integrity test.
For more information, please see Pall Publications:
The Water Intrusion Test is a practical and validated test which can be used for in-situ integrity testing of hydrophobic gas filters. This test is conducted with deionized or higher quality water, without the need for low-surface-tension flammable solvents (such as Isopropyl Alcohol or Ethanol). Due to occupational risks, environmental regulations, safety guidelines and cost associated with handling these low surface tension solvents, water intrusion is becoming the method of choice for integrity testing hydrophobic microbial rated filters for air or gas applications.
Water Intrusion testing is the preferred test where the hydrophobic microbial rated gas filter:
- Is integrity tested in-situ
- A pre-use test is performed (especially after sterilization)
- Alcohol use is restricted or not allowed in the production area
The Forward Flow or Bubble Point test, is the preferred method to integrity test filters in applications where:
- A small area filter is used
- An off-line filter integrity test is performed
- To confirm filter integrity following a water intrusion test failure evaluation
For more information, please see Pall Publication, USD 3033 Application Note: Best Practices for Successful Filter Integrity Testing Using the Water Intrusion Test (WIT) Method
To successfully perform a Water Intrusion Test (WIT), the entire length of the filter has to be covered by (submerged in) water for the duration of the test.
During pressurization of the filter housing assembly to the water intrusion test pressure, the water level drops upstream of the filter. The reason is due to compression of the membrane pleats and elimination of gas bubbles during initial pressurization.
This can leave a portion of the filter exposed to pressurized air, which will freely flow through the exposed area and result in a false test failure.
To troubleshoot the cause of a test failure, Pall recommends:
· To refill the filter housing with water and repeat the test, or
· Before the test, increase the upstream volume, to have more water above the filter, to compensate for the reduced water levels resulting from the compression.
For more information, please see Pall Publication, USD 3033 Application Note: Best Practices for Successful Filter Integrity Testing Using the Water Intrusion Test (WIT) Method
There is no general rule to this and depends on the filter application.
The user should qualify the filter change-out frequency for their specific application, but this should never exceed Pall claims for cumulative sterilization cycles and/or the maximum differential pressure. These specificationswere established by Pall in controlled laboratory conditions with filters that were not exposed to any process (including manufacturing or production) conditions.
The filter lifetime and change-out should be based on a risk assessment by the user for their specific application including relevant validation/qualification data.
For example, the impact of a post-use integrity test failure of the filter should be considered.
For gas filtration in compressed gas, tank vent, or utilities setting, industry best practice is to replace the filter under a pre-defined preventative maintenance schedule (i.e. at a minimum of 12-month cycle).
For critical applications, single use is recommended to eliminate the risks of cross contamination between batches.
For more information, please see Pall Publication:
For gas filtration in a vent application, YES, Pall sterilizing grade gas filters can be used in both directions.
For gas filtration, Pall offers the following sterilizing grade gas filters:
Emflon® II V002PV
Emflon PFR
Emflon HTPFR
Acro® 50 (6074270)
Acrodisc® KM292HP
These filter devices feature a symmetrical filter media construction, where two layers of membrane of the same pore rating are used in the manufacturing of the final filter cartridge or capsule.
When a filter of a symmetrical media construction is used for venting purposes, in either forward or reverse flow direction, the gas flow and any contaminants will travel through a torturous path of the same characteristic and length as in the forward direction. This will lead to the same retention efficiency in either flow direction.
Based on the symmetric media construction the filter will thus act as a sterile barrier when venting in either flow direction. Therefore, Pall Emflon® II V002PV, Emflon PFR, Emflon HTPFR, Acro® 50 (6074270) and Acrodisc® KM292HP filters can be used for bi-directional flow applications under this venting mode of filtration.
In order to consider the influence of the temperature, each filter integrity test method needs to be treated independently, as they are based on different physical principles.
Forward Flow
Forward Flow integrity test limits issued for Pall filters, when wet with standard wetting fluids such as water, 60/40 IPA or similar solutions, apply to a test temperature of 20 °C ± 5 °C.
Any variation in the temperature of any gas volume in the filter test assembly during the measurement phase has an effect on the flow measurement. Most integrity test instruments measure Forward Flow on the upstream side of filter, as a function of gas pressure change1. Variations in temperature during the test lead to expansion or compression of the gas in the test assembly (tubing and housing upstream of the filter). Such variations of the gas volume may lead to inaccurate flow measurements. Therefore, it is recommended to keep the temperature of the filter assembly constant during the test period.
Pall recommends that the temperature of the filter assembly during the test should not vary more than ± 1 °C. Some simple ways to accomplish this are listed below.
Bubble Point
Changes of surface tension are of direct relevance for bubble point testing as the surface tension influences the capillary forces holding the wetting liquid in the membrane pores. The measured bubble point of a given filter which is fully wetted will increase and decrease in direct proportion with the surface tension of the wetting liquid.
This means that lower bubble point values will be measured at higher temperature, and higher bubble point values will be measured at lower temperature.
Water Intrusion Test (WIT)
For Pall filters, the water intrusion limit values apply to ambient temperature (20 °C) with a specified range of ± 2 °C. During the test period, the temperature of the filter assembly should not vary more than ± 1 °C.
For the WIT, the temperature of the gas in the filter assembly will have the same effects on the gas volume as indicated above for the Forward Flow test. Water temperature will also have an effect on the measurements.
As the WIT measures evaporative flow, which is typically much lower than diffusion measurements (as measured by the Forward Flow test), any temperature changes will have a greater effect on the WIT measurements compared to the Forward Flow test and may not be identified by environmental or assembly temperature monitoring.
Maintaining Constant Temperature During Integrity Testing
The following is a list of approaches to maintaining a constant temperature during integrity testing:
- Acclimate the filter and fluids to room temperature before starting the test. This is especially important for the WIT: Our guidance is to dispense the water into a container and acclimate for >4 hours.
- Avoid placing the filter assembly under heating or cooling registers.
- Avoid handling the filter during the test.
- In cases where room temperature fluctuates, it may be necessary to insulate tubing and filter assembly.
1 The Palltronic Flowstar line of integrity test instruments measure Forward Flow by direct measurement. The impact of a change in temperature during the measurement (“Test”) phase will result in an unstable flow measurement, which will extend the test time.
Pall Corporation’s approach for testing multi-modular, sterilizing-grade filter assemblies is to provide assembly-specific Forward Flow limit values below the sum of the maximum allowable FF limit values for the individual filter modules (i.e. a multiplying or reducing factor). This reduction in allowable Forward Flow is designed to reduce the risk that a marginal filter test failure (in the unlikely event that one is present) cannot be detected in a multi-element assembly. It results in a tighter, more conservative test limit when compared to a linear multiplier.
While the use of multiplying factors is not a regulatory requirement, it is Pall Corporations’ philosophy on integrity testing to use practices that provide the maximum safety for large filter area installations. This needs to be balanced with the risk that a set of integral modules may fail the test due to the application of a multiplying factor that is too stringent (“false fail”). This approach is also described in the 2008 revision of PDA Technical Report No. 26 on Sterilizing Filtration of Liquids 1.
Pall Corporation’s basis for defining the appropriate multiplying factor for a specific multi-modular installation is based on several parameters including:
- Type of membrane and number of elements
- Statistical distribution of observed Forward Flow (FF) values for the specific filter
- Mean deviation for Forward Flow distribution
- Standard deviation of Forward Flow distribution
- Point of first failure during microbial challenge (if observed)
Forward Flow limits obtained from Pall Corporation for multi-element filter assemblies will incorporate the appropriate multiplier.
PDA Journal of Pharmaceutical Science and Technology, Technical Report No. 26 Sterilizing Filtration of Liquids, Rev. 2008. Supplement Volume 62, No. S-5
Prior to performing an integrity test, it is imperative that the pore structure of the filter membrane be filled with the wetting fluid. To assure complete wetting, Pall recommends applying downstream flow restriction (sometimes referred to as back pressure, not to be confused with reverse pressure) during the filter flush.
The use of a downstream flow restriction helps to:
- Ensure uniform flow distribution through the entire length of the filter
- Overcome the tendency for fluid to flow through the path of least resistance.
- Remove air entrapped in the membrane pleats, by further solubilizing the air (due to increased pressure in the system) and by compressing air bubbles to a size where they can freely pass through the membrane.
When testing filters larger than 254 mm (10 in.) or multi-round systems, a downstream flow restriction will ensure the fluid reaches the top of the housing during venting. Otherwise, the hydrostatic pressure of the liquid will cause it to flow out of the downstream side of the housing before reaching the vent at the highest point in the system.
To apply a downstream flow restriction, Pall recommends installing a pressure gauge downstream of the filter housing, followed by a flow control valve such as a diaphragm valve.
For more information, please see Pall Publication, USD3297_User Guide: Wetting and Flushing of Pall Microbially_Rated_Filter Cartridges and Capsules
Pall manufactures different membrane filters for gas filtration. We use either Polytetrafluoroethylene (PTFE) filter membrane or Polyvinylidene Fluoride (PVDF) modified, for such a purpose.
In our Pall Allegro™ single-use systems, the method to sterilize these systems is by gamma irradiation.
The Polytetrafluoroethylene (PTFE) filter membrane, is incompatible with gamma irradiation, so it could not be used as a gas filter in our Single-Use Systems.
The Polyvinylidene Fluoride (PVDF) modified is compatible with gamma irradiation and for this reason is the device to be used with our Single-Use-Systems.
Pall Emflon II V002 sterilizing grade filters uses a filter membrane made of polyvinylidene difluoride (PVDF).
Integrity test failures can be caused by a range of root causes, some of which are listed below:
- System leaks (filter housing, fittings, tubes, etc.)
- Insufficient test time
- Incorrect temperature of wetting fluid
- Insufficient stabilization time
- Temperature influence
- Incomplete wetting of the filter
- Incorrect test limits
- Incorrect pressure source
- Filter defects or damage
- Compromised O-ring seal
- Incorrect filter selection
When an integrity test failure occurs, the first step is to verify the system setup and test parameters. After all of these conditions have been verified, the filter should be re-wet and tested again (using the Forward Flow test, even if the initial failure was recorded using a bubble point test, except for small area filters (<200cm2).
If the result is a pass, then the filter is integral. If it fails, the Forward Flow test should be repeated after a more vigorous wetting step. This can include a larger flush volume, application of back pressure (downstream flow restriction), or increased differential pressure. If the filter fails again, a flush and Forward Flow integrity test with a low surface tension wetting fluid, such as 60:40 IPA/water, should be performed. If the filter fails the integrity test again, the filter (still installed in the housing if possible) should be returned to Pall Corporation for further analysis.
When investigating an integrity test failure, one of the first steps is to determine if any leaks are present in the system under test. Integrity test instruments perform the measurements on the upstream side of the filter, thus preserving the downstream sterility of the system. The test instrument is not able to differentiate gas flow through the filter from gas flow through a leak in the hardware.
Prior to performing the leak test, a visual inspection of the system is recommended to identify any potential leak sources. Damage to the housing or to the o-ring seals are common sources of leaks. Such signs of damage include cuts on the O-rings, or misshapen or dented housings.
Pall recommends performing a leak test using the Palltronic® Flowstar® IV, as follows:
- The leak test can be performed on a filtration system with or without a filter installed. If a filter is installed, as is the case with capsule filters, the filter must be dry. Diffusive or bulk gas flow through a wet filter cannot be differentiated from leaks in the assembly.
- The outlet of the housing should be sealed with either a blank endcap, valve, or other suitable termination method.
- All drain and vent valves must be in the closed position, except in cases where the connection to the instrument is made through a vent.
- A length of pneumatic tubing with the Palltronic Flowstar IV external vent valve installed should be used to connect the OUT port of the Palltronic® Flowstar IV integrity test instrument to the housing vent port.
- From the Palltronic Flowstar IV main menu, select the “Leak Test” function.
- The Forward Flow test pressure that is used to test the filters should be used as the pressure for the leak test.
- The maximum system size for the leak test is 50 liters.
If the Palltronic Flowstar IV integrity test instrument is not able to detect a pressure loss in the volume or the leak rate is too small to be detected, it will report “no leak detectable” or “flow within limits’ as the result. These results confirm that the filter system under test is free of significant upstream leaks.
If the test reports “flow outside limits” (> 1 mL/min)
- Ensure that the test is being carried out under stable temperature conditions.
- Re-check the connections and housing enclosure are tightened fully.
- If sanitary fittings are an integral part of the filter housing, then the elastomeric seals should be examined and changed if necessary.
- For smaller housings, submerging the pressurized system in a water bath and looking for bubbles will indicate the location of the leak.
- A soap-based leak detecting product can be used around the fittings and connections to locate a leak.
For more information, please contact Pall’s NEW Equipment Support Hotline.
The Bubble Point test measures the pressure region at which diffusive flow transitions into bulk flow, by looking for a deviation from the stable background flow versus flow through open pores. However, a marginal leak in the upstream side of the system under test, or a minor damage to the filter, can add a small amount of gas flow. This could be interpreted by the test instrument as part of the background flow, and therefore, the leak would not be detected. The module factor has been designed into the software of the Palltronic® Flowstar IV software to detect these types of flow contributions.
As stated in USD 2594, Instructions for use for the Palltronic® Flowstar IV integrity test instrument, the Module Factor (MF) defines the sensitivity for the Leak Test phase which is executed at the beginning of the Bubble Point test sequence.
The Bubble Point integrity test using the Flowstar IV integrity test instrument features this stabilization/leak test before proceeding to the actual step-wise increase of pressure for the “Bubble Point” test. This initial leak test seeks to establish that filter system under test shows an expected and typical background flow.
For this test, the upstream side of the filter is pressurized to 80% of the minimum programmed BP value, followed by a measurement of the gas flow occurring at this gas pressure, which is compared against a limit value represented by the programmed filter type related ‘Module Factor’. If the gas flow measured is below the limit value, the BP test proceeds.
The lower the “Module factor” is set, the more sensitive it will be to detect a deviation from the typical background flow for the filter under test during this step in the test sequence.
The expected, typical and thus acceptable background flow caused by gas diffusion is dependent on the filter area. Pall recommends that the “Module Factor” should be set to the number of 10” modules being tested. It should be set to 1.0 for 10” cartridges, 0.5 for 5” cartridges (e.g. AB05 or SLK7002), 0.2 for smaller (e.g. AB02 or SLK7001) filter and 0.1 for flat membranes (e.g. 142 mm discs) or mini capsules (KA02 or similar). The default value is 1 if nothing is entered.
This will ensure that the test sequence is aborted when a filter clearly displays a gas flow above the expected typical background value.
For software versions > 2.0b, the user can set the Module Factor to as low as 0.01 for small area filters such as the Acro 25/50 and KM5 devices. See Table I below for the recommended input values for the module factor.
For more information, please contact Pall’s NEW Equipment Support Hotline.
The Bubble Point specification provided on the Certificate of Test is a manufacturing specification for the filter membrane and should not be used by the end-user as a test limit for the final device. The Manufacturing specifications limit value relies on a defined endpoint and is therefore has a defined end-point. This uses a different Bubble Point test method than what the end-user performs (which inherently is more subjective).
Pall provides a certificate of test with each sterilizing grade filter. The first paragraph Certificate of Test discusses the membrane Bubble Point. An example of this statement is as follows: The 0.2 µm filter membrane used in the filter element has a quantitative bubble point (i.e. "KL") which met or exceeded 3655 mbar (53.0 psi) in water.
As stated, this value is the minimum specification of the flat sheet membrane that is used in the device, not the specification of the device itself. This test, termed the Quantitative Bubble Point (QBP), is objectively and quantitatively defined as the pressure needed to achieve a specified air flow limit through a specified membrane area. This test is conducted on samples from every sterilizing grade membrane roll produced by Pall and reflects the largest pores in each entire roll, to be used either in discs or in cartridge production.
The minimum expected Bubble Point (BP) limit provided for cartridge filters are typically lower than the minimum membrane QBP. The lower end-user Bubble Point limit considers the lower observed Bubble Point typically seen with increasing filter area. It also considers the variability in BP measurements due different algorithms used by different test instruments, as well as variability due to human interpretation of the test result when a visual (manual) Bubble Point test is used.
Please contact Pall Corporation if you require minimum Bubble Point test parameters.