Scalable Purification of Adenovirus Serotype 5 using Mustang® Q Membrane
Heather Mallory, Brian Gardell, Todd Sanderson, Amanda Rose and Rachel Legmann
INTRODUCTION
- Due to the growing interest in the use of viruses for gene therapy and vaccines many virus-based products are being developed.
- The manufacturing process for viruses poses new challenges both for process development and for regulatory authorities tasked with assuring quality, efficacy and safety of the final product. For each process, the design of a suitable purification strategy will depend on many variables including the vector production system and the nature of the virus.
- A case study will be presented here to illuminate some challenges and solutions associated with downstream purification process development.
- Adenovirus is used in this study as part of a therapy for Type 1 diabetes. Currently, diabetes affects more than 400 million people worldwide and causes 3 - 4 million deaths annually. To restore insulin production capability to patients with Type 1 diabetes, Orgenesis♦ is using a cell therapy approach. This approach manipulates a diabetic patient’s own liver cells outside of the body (ex vivo) via viral transduction with three transgenes, each delivered by adenovirus serotype 5 (Ad5). Traditionally, this virus is purified using Cesium Chloride (CsCl) gradient centrifugation steps. Aside from being slow and cumbersome, the CsCl gradient centrifugation purification method is not scalable. To adapt purification of adenovirus to a practical, manufacturing platform we have developed a purification process which uses AEX membrane chromatography. For these studies, a total of 2 x 1012, 4 x 1012 and 6 x 1012 Infectious Units (IFU) of excreted hPDX-1, hNeuroD and hMafA Ad5 respectively was generated in each Toxicology (Tox) run in the iCELLis® Nano bioreactor.
PURPOSE
Develop an industrialized adenoviral vector purification process using Mustang Q ion-exchange membrane chromatography
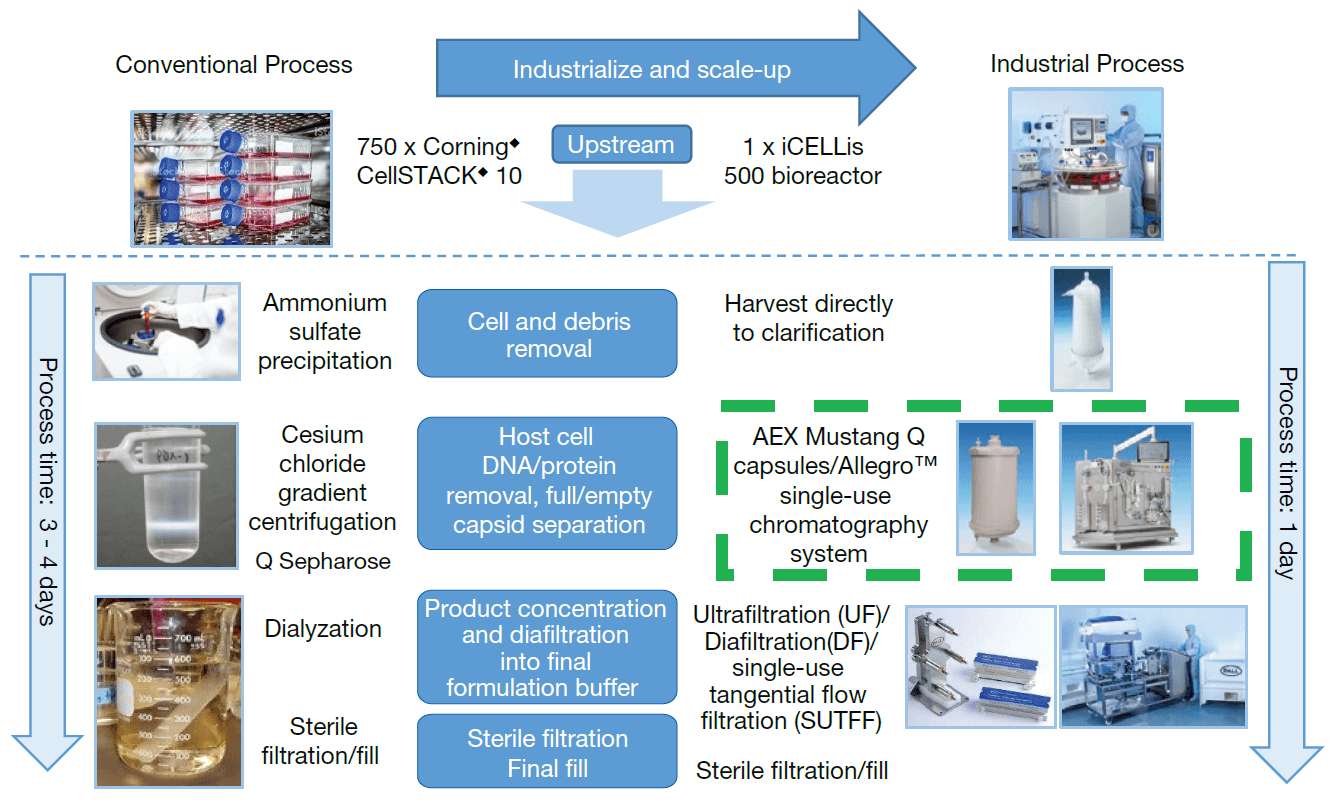
To go to clinical trials for trans-differentiation of liver cells, Orgenesis needs to produce purified Ad5 at an industrial scale. This goal was unachievable with the current cesium chloride (CsCl) gradient centrifugation process (Figure 1) as it is time consuming and not scalable. Pall has developed an industrial scale purification process using Pall depth filters, low shear Pall Mustang membrane chromatography and a Pall tangential flow filtration (TFF) membrane process which is scalable and low shear, which minimizes damage to the virus. The process shown here was then scaled up from less than 1 L to 1300 L (Figure 2).
Figure 1
Industrialize and scale-up adenovirus purification
The green box shown in the figure indicates ion-exchange Mustang Q membrane purification step which is the main focus of this case study. Mustang Q membrane is used to replace the conventional cesium chloride purification step, that requires 50 incubators and 5 X more FTEs. The industrialized purification process takes less than a day to complete at large scale. This is in comparison to more than 4 - 5 days completion time for the conventional limited scale centrifugation process.
Figure 2
Downstream process development and scalability strategy
A. Purification industrial process map
B. 1000 x linear scalability of Mustang Q membrane from development scale of 1.4 L to manufacturing scale of 1300 L
MATERIALS AND METHODS
- Cells: HEK293 (ATCC) producing cells were used for amplification of three adenovirus serotype 5 containing different transgenes (hhPDX-1, hNeuroD and hMafA provided by Orgenesis). Cells were grown for four days in DMEM with 8% serum to a target density of 2.5 - 3.0 x 105 cells/cm2 . A full media exchange was performed and the cells were infected with a Multiplicity of Infection (MOI) of 20. Vector was harvested from the bioreactor in the spent media fraction.
- Virus standard: The virus standard used in these experiments was purified using conventional CsCl density method (provided by Orgenesis).
- Viral vectors were produced using Pall’s iCELLis bioreactor systems of varying sizes.
- Small scale runs were performed in the iCELLis Nano 0.53 m2 , iCELLis Nano 1.07 m2 , iCELLis Nano 2.65 m2 , and iCELLis Nano 4 m2 bioreactor systems (Pall part number (P/N) 810039NS, 810061NS, 810206NS, and 810042NS, respectively).
- Large-scale runs were performed in the iCELLis 500-66 m2 bioreactor system (Pall P/N 4415-l500V66).
- Harvest was clarified using a Pall V100P Supracap™ 100 depth filter capsule (Pall P/N NP5LV1001) followed by 0.2 µm filtration using Pall’s Supor® EKV in a Pall Mini Kleenpak™ capsule (Pall P/N KM5EKVP2S).
- Mustang Q membrane capsule (Pall P/N CL3MSTGQP1) was used for bind/elute anion exchange chromatography. Prior to purification, the Mustang Q capsule is sanitized/hydrated using 0.5 M NaOH, then charged with a 1 M NaCl, 20 mM sodium phosphate pH 7.3 and equilibrated using a 20 mM sodium phosphate buffer with 150 mM NaCl pH 7.3 and conductivity of 17 mS/cm.
- Clarified material from HEK 293 cell media harvest with a pH of 7.2 - 7.4 and a conductivity of 12 - 18 mS/cm is loaded onto the Mustang Q membrane capsules, which were used in bind/elute mode to purify Ad5 from host-cell proteins and host-cell DNA. A series of salt solutions at different concentrations was used prior to the elution. Once the virus was eluted from the membrane, UF/DF was started immediately to concentrate and exchange Ad5 out of the high salt buffer into final formulation buffer.
- UF/DF: Purified material was then subjected to UF/DF using a Pall Cadence™ diafiltration module with 100 kDa molecular weight cut-off (MWCO) Omega™ membranes (Pall P/N CSUM100T001).
- Analytics: pH and conductivity checks were performed using an off-line Mettler Toledo♦ seven excellence pH meter, the Adeno-X♦ rapid titer kit, the HEK 293 HCP ELISA assay, and the Quant-iT♦ dsDNA assay.
RESULTS AND DISCUSSION
Ion-exchange Mustang Q membrane chromatography viral vector purification development in small scale volumes.
Small scale runs were initially done using Ad5 containing the hPDX-1 transgene to determine: 1. Membrane load capacity, 2. Optimal wash profile and, 3. Elution condition which achieves highest yield, with lowest non-product impurities such as HCP, residual DNA and empty capsids.
Development scale run using Mustang Q membrane in bind/elute mode.
To clear less tightly bound impurities prior to elution, wash steps were chosen at 150 mM and then 10 mM NaCl (low salt conditions to try and knock off any hydrophobically bound material bound to the virus from the virus. This step was then followed by 300 mM, and 500 mM NaCl wash steps.
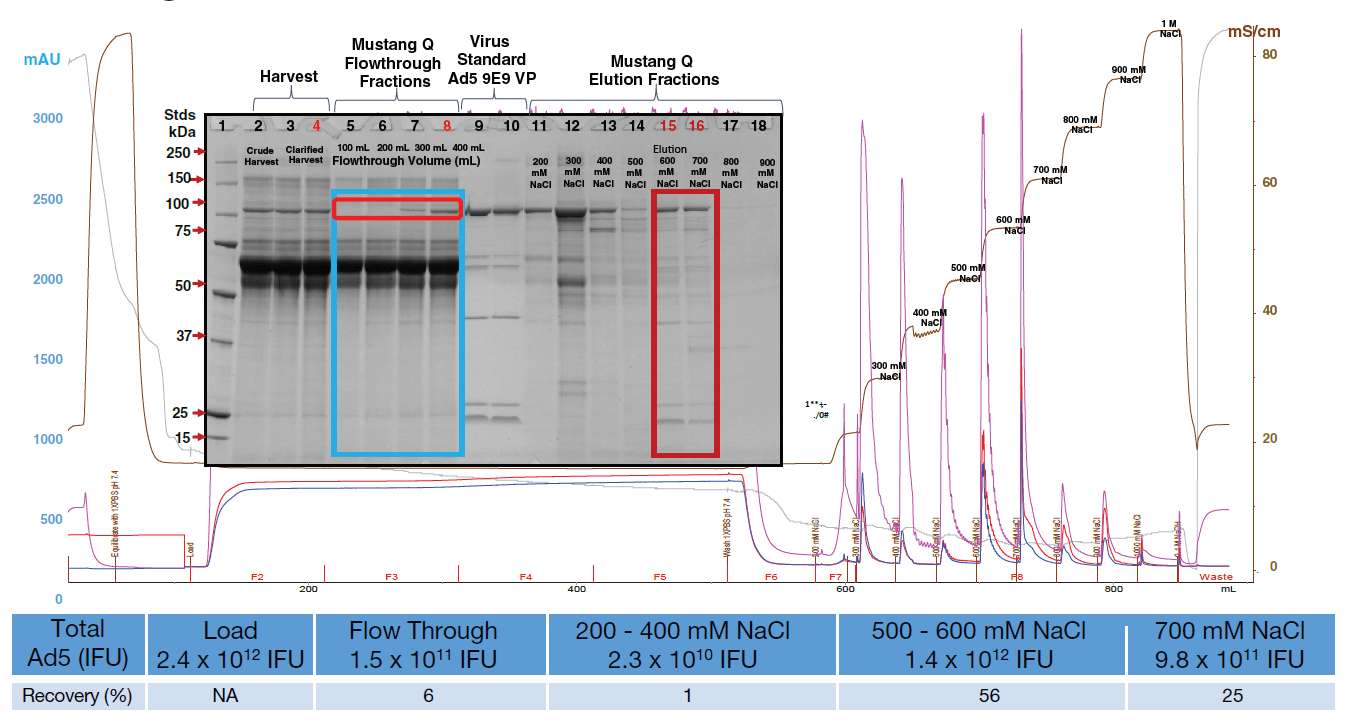
Fractions of the flowthrough from development runs were collected to determine where breakthrough occurred based on visualization of hexon protein on the SDS-PAGE. Results for infectious activity were then checked using the Adeno-X activity assay. Data showed that the majority of the infectious adenovirus is found in the 650 mM NaCl elution fraction from the Mustang Q device with 96% recovery. Therefore the final elution conditions chosen were 650 mM NaCl (58 – 61 mS/cm).
Figure 3
Chromatography profile for development scale run using Mustang Q membrane in bind/elute mode
Fractions of the flowthrough were collected to determine where breakthrough occurred based on visualization of hexon protein on the SDS-PAGE. Results were then confirmed with Adeno-X activity assay. It is clear from this data that the majority of the infectious adenovirus is in the 650 mM NaCl elution fraction from the Mustang Q device with 96% recovery. Final elution conditions chosen were 650 mM NaCl (58 - 61 mS/cm) following wash steps at 150 mM, 10 mM, 300 mM and 500 mM NaCl.
Table 1
Purification of Ad5 by Mustang Q membrane providing impurities clearance
Purified adenoviral vectors for Tox material study.
- The Mustang Q membrane purifies virus from clarified harvest in a single bind/elute chromatography step and provides greatest clearance of HCP and hcDNA from adenovirus for this process.
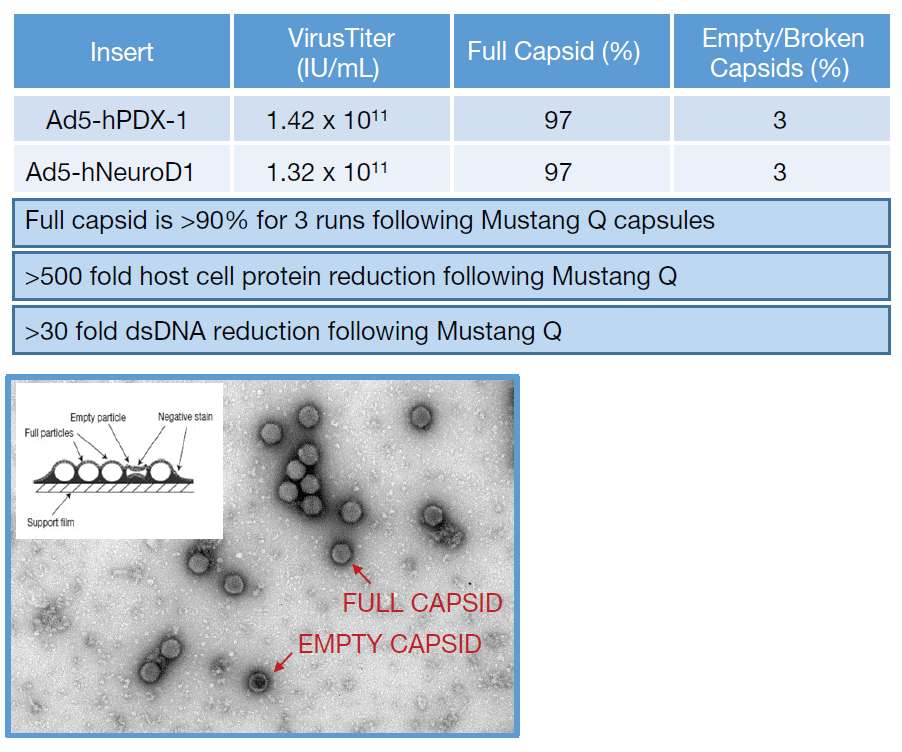
- Purification steps provided ≥78% yield, >500 x reduction of serum and host cell protein, >30 x reduction of hcDNA and high ratio of full vs. empty capsid (>90%).
- For the production of toxicological material, three runs were performed in the iCELLis Nano and purified with a Mustang Q CL3MSTGQP1 device (60 mL bed volume). This device was loaded with clarified HEK293 cell supernatant, which is in DMEM with 8% serum (no lysis step) at pH 7.3, 12-18 mS/cm.
- Clearance of serum protein, host cell protein and host cell DNA was provided through the Mustang Q step. Based on results from the adenovirus assay, recovery was greater than 75% for all Tox animal runs.
- Purified adenovirus produced from Tox runs was used in a Tox study and this Ad5 material demonstrated comparable results to the Ad5 material produced using a CsCl gradient centrifuged conventional non-industrial scale process.
CONCLUSIONS
- Equivalent vector purity and yield of recovery obtained using Mustang Q membrane chromatography compared to CsCl purification method.
- This study demonstrates successful development of a simplified industrial scale purification of adenovirus with a 1 day process time compared with 3 days for the conventional CsCl gradient centrifugation process.
- Infective virus particles were efficiently purified from clarified lysate using a single membrane chromatography step in less than 4 hours.
- The Mustang Q membrane chromatography step provides a processing time of less than 4 hours. The greatest process clearance for both HCP and dsDNA is provided by the Mustang Q membrane bind/elute step.
- Overall process recovery was greater than 65%.
- Remove > 500 x and 30 x host cell protein and dsDNA respectively.
- Enrich for more than 90% full capsids.
- Functionality and purity of final product is comparable to conventional flatware process.
- Manufacturing process is easy to implement and facilitates high titer adenoviral vector production for preclinical and clinical material.
Maximize Speed to Market
Flexibility of Single-Use for Gene Therapy Manufacturing